Neuroprotective Effects of Thymol, a Dietary Monoterpene Against Dopaminergic Neurodegeneration in Rotenone-Induced Rat Model of Parkinson’s Disease
Abstract
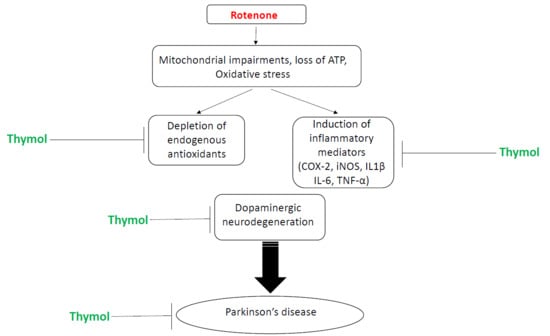
:1. Introduction
2. Results
2.1. Thymol Preserved TH+ Dopaminergic Neurons in SNc Regions and Dopaminergic Fibers in Striatum Regions of the Brain
2.2. Thymol Inhibited Lipid Peroxidation and Restored GSH and Endogenous Enzymes Activity
2.3. Thymol Inhibited Activation of Glial Cells
2.4. Thymol Attenuated Activation of Proinflammatory Cytokines
2.5. Thymol Attenuated Expression Levels of COX-2 and iNOS
3. Discussion
4. Materials and Methods
4.1. Drugs and Chemicals
4.2. Experimental Animals
4.3. Experimental Design
4.4. Tissue Collection
4.5. Sample Preparation for Biochemical Studies
4.6. Assessment of Lipid Peroxidation and Glutathione
4.7. Assessment of Antioxidant Enzymes Activity
4.8. Estimation of Proinflammatory Cytokines
4.9. Immunohistochemistry of Tyrosine Hydroxylase (TH)
4.10. Immunofluorescence Staining of GFAP and Iba-1
4.11. Determination of Activated Astrocytes and Microglia in the Striatum
4.12. Western Blot Analysis of COX-2 and iNOS
4.13. Protein Estimation
4.14. Statistical Analyses
5. Conclusions
Author contributions
Funding
Conflicts of Interest
References
- Cacabelos, R. Parkinson’s Disease: From Pathogenesis to Pharmacogenomics. Int. J. Mol. Sci. 2017, 18, 551. [Google Scholar] [CrossRef]
- Van Bulck, M.; Sierra-Magro, A.; Alarcon-Gil, J.; Perez-Castillo, A.; Morales-Garcia, J.A. Novel Approaches for the Treatment of Alzheimer’s and Parkinson’s Disease. Int. J. Mol. Sci. 2019, 20, 719. [Google Scholar] [CrossRef]
- Moreira, P.I.; Zhu, X.; Wang, X.; Lee, H.G.; Nunomura, A.; Petersen, R.B.; Perry, G.; Smith, M.A. Mitochondria: A therapeutic target in neurodegeneration. Biochem. Biophys. Acta 2010, 1802, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Glass, C.K.; Saijo, K.; Winner, B.; Marchetto, M.C.; Gage, F.H. Mechanisms underlying inflammation in neurodegeneration. Cell 2010, 140, 918–934. [Google Scholar] [CrossRef] [PubMed]
- Mander, P.; Brown, G.C. Activation of microglial NADPH oxidase is synergistic with glial iNOS expression in inducing neuronal death: A dual-key mechanism of inflammatory neurodegeneration. J. Neuroinflamm. 2005, 2, 20. [Google Scholar] [CrossRef]
- Sanchez-Pernaute, R.; Ferree, A.; Cooper, O.; Yu, M.; Liisa Brownell, A.; Isacson, O. Selective COX-2 inhibition prevents progressive dopamine neuron degeneration in a rat model of Parkinson’s disease. J. Neuroinflamm. 2004, 1, 6. [Google Scholar] [CrossRef]
- Hirsch, E.C.; Breidert, T.; Rousselet, E.; Hunot, S.; Hartmann, A.; Michel, P.P. The role of glial reaction and inflammation in Parkinson’s disease. Ann. N. Y. Acad Sci. 2003, 991, 214–228. [Google Scholar] [CrossRef]
- Kujawska, M.; Jodynis-Liebert, J. Polyphenols in Parkinson’s Disease: A Systematic Review of In Vivo Studies. Nutrients 2018, 10, 642. [Google Scholar] [CrossRef] [PubMed]
- Shahpiri, Z.; Bahramsoltani, R.; Hosein Farzaei, M.; Farzaei, F.; Rahimi, R. Phytochemicals as future drugs for Parkinson’s disease: A comprehensive review. Rev. Neurosci. 2016, 27, 651–668. [Google Scholar] [CrossRef] [PubMed]
- Nagoor Meeran, M.F.; Javed, H.; Al Taee, H.; Azimullah, S.; Ojha, S.K. Pharmacological Properties and Molecular Mechanisms of Thymol: Prospects for Its Therapeutic Potential and Pharmaceutical Development. Front. Pharmacol. 2017, 8, 380. [Google Scholar] [CrossRef]
- Yanishlieva, N.V.; Marinova, E.M.; Gordon, M.H.; Ranevaa, V.G. Antioxidant activity and mechanism of action of thymol and carvacrol in two lipid systems. Food Chem. 1999, 64, 59–66. [Google Scholar] [CrossRef]
- Aeschbach, R.; Loliger, J.; Scott, B.C.; Murcia, A.; Butler, J.; Halliwell, B.; Aruoma, O.I. Antioxidant actions of thymol, carvacrol, 6-gingerol, zingerone and hydroxytyrosol. Food Chem. Toxicol. 1994, 32, 31–36. [Google Scholar] [CrossRef]
- Zahin, M.; Ahmad, I.; Aqil, F. Antioxidant and antimutagenic activity of Carum copticum fruit extracts. Toxicol. In Vitro 2010, 24, 1243–1249. [Google Scholar] [CrossRef] [PubMed]
- Ozen, T.; Demirtas, I.; Aksit, H. Determination of antioxidant activities of various extracts and essential oil compositions of Thymus praecox subsp. skorpilii var. skorpilii. Food Chem. 2011, 124, 58–64. [Google Scholar] [CrossRef]
- Karpanen, T.J.; Worthington, T.; Hendry, E.R.; Conway, B.R.; Lambert, P.A. Antimicrobial efficacy of chlorhexidine digluconate alone and in combination with eucalyptus oil, tea tree oil and thymol against planktonic and biofilm cultures of Staphylococcus epidermidis. J. Antimicrob. Chemother. 2008, 62, 1031–1036. [Google Scholar] [CrossRef] [Green Version]
- Littlejohn, D.; ManFano, E.; Clarke, M.; Bobyn, J.; Moloney, K.; Haylay, S. Inflammatory mechanisms of neurodegeneration in toxin-based models of Parkinson’s disease. Parkinsons Dis. 2010, 2011, 713517. [Google Scholar]
- Cannon, J.R.; Tapias, V.; Na, H.M.; Honick, A.S.; Drolet, R.E.; Greenamyre, J.T. A highly reproducible rotenone model of Parkinson’s disease. Neurobiol. Dis. 2011, 34, 279–290. [Google Scholar] [CrossRef]
- Sherer, T.B.; Betarbet, R.; Kim, J.H.; Greenamyre, J.T. Selective microglial activation in the rat rotenone model of Parkinson’s disease. Neurosci. Lett. 2003, 341, 87–90. [Google Scholar] [CrossRef]
- Betarbet, R.; Sherer, T.B.; MacKenzie, G.; Garcia-Osuna, M.; Panov, A.V.; Greenamyre, J.T. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat. Neurosci. 2000, 3, 1301–1306. [Google Scholar] [CrossRef]
- Saravanan, K.S.; Sindhu, K.M.; Senthilkumar, K.S.; Mohanakumar, K.P. L-deprenyl protects against rotenone-induced, oxidative stress-mediated dopaminergic neurodegeneration in rats. Neurochem. Int. 2006, 49, 28–40. [Google Scholar] [CrossRef]
- Inden, M.; Kitamura, Y.; Tamaki, A.; Yanagida, T.; Shibaike, T.; Yamamoto, A.; Takata, K.; Yasui, H.; Taira, T.; Ariga, H.; et al. Neuroprotective effect of the antiparkinsonian drug pramipexole against nigrostriatal dopaminergic degeneration in rotenone-treated mice. Neurochem. Int. 2009, 55, 760–767. [Google Scholar] [CrossRef]
- Fujikawa, T.; Kanada, N.; Shimada, A.; Ogata, M.; Suzuki, I.; Hayashi, I.; Nakashima, K. Effect of sesamin in Acanthopanax senticosus HARMS on behavioral dysfunction in rotenone-induced parkinsonian rats. Biol. Pharm. Bull. 2005, 28, 169–172. [Google Scholar] [CrossRef]
- Javed, H.; Azimullah, S.; Haque, M.E.; Ojha, S.K. Cannabinoid Type 2 (CB2) Receptors Activation Protects against Oxidative Stress and Neuroinflammation Associated Dopaminergic Neurodegeneration in Rotenone Model of Parkinson’s Disease. Front. Neurosci. 2016, 10, 321. [Google Scholar] [CrossRef]
- Ojha, S.; Javed, H.; Azimullah, S.; Haque, M.E. β-Caryophyllene, a phytocannabinoid attenuates oxidative stress, neuroinflammation, glial activation, and salvages dopaminergic neurons in a rat model of Parkinson disease. Mol. Cell. Biochem. 2016, 418, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Tansey, M.G.; Goldberg, M.S. Neuroinflammation in Parkinson’s disease: Its role in neuronal death and implications for therapeutic intervention. Neurobiol. Dis. 2010, 37, 510–518. [Google Scholar] [CrossRef]
- Gerhard, A.; Pavese, N.; Hotton, G.; Turkheimer, F.; Hammers, M.; Es, A.; Eggert, K.; Oertel, W.; Banati, R.B.; Brooks, D.J. In vivo imaging of microglial activation with [11C] (R)-PK11195 PET in idiopathic Parkinson’s disease. Neurobiol. Dis. 2006, 21, 404–412. [Google Scholar] [CrossRef]
- Chen, G.; Liu, J.; Jiang, L.; Ran, X.; He, D.; Li, Y.; Huang, B.; Wang, W.; Fu, S. Galangin Reduces the Loss of Dopaminergic Neurons in an LPS-Evoked Model of Parkinson’s Disease in Rats. Int. J. Mol. Sci. 2017, 19, 12. [Google Scholar] [CrossRef] [PubMed]
- Azizi, Z.; Ebrahimi, S.; Saadatfar, E.; Kamalinejad, M.; Majlessi, N. Cognitive enhancing activity of thymol and carvacrol in two rat models of dementia. Behav. Pharmacol. 2012, 23, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Jenner, P.; Olanow, C.W. Oxidative stress and the pathogenesis of Parkinson’s disease. Neurology 1996, 47, S161–S170. [Google Scholar] [CrossRef] [PubMed]
- Pohl, F.; Kong Thoo Lin, P. The Potential Use of Plant Natural Products and Plant Extracts with Antioxidant Properties for the Prevention/Treatment of Neurodegenerative Diseases: In Vitro, In Vivo and Clinical Trials. Molecules 2018, 23, 3283. [Google Scholar] [CrossRef]
- Wojdylo, A.; Oszmianski, J.; Czemerys, R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007, 105, 940–949. [Google Scholar] [CrossRef]
- Barone, F.C.; Feuerstein, G.Z. Inflammatory Mediators and Stroke: New Opportunities for Novel Therapeutics. J. Cereb. Blood Flow Metab. 1999, 19, 819–834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sita, G.; Hrelia, P.; Tarozzi, A.; Morroni, F. Isothiocyanates Are Promising Compounds against Oxidative Stress, Neuroinflammation and Cell Death that May Benefit Neurodegeneration in Parkinson’s Disease. Int. J. Mol. Sci. 2016, 17, 1454. [Google Scholar] [CrossRef]
- Song, I.U.; Kim, Y.D.; Cho, H.J.; Chung, S.W. Is Neuroinflammation Involved in the Development of Dementia in Patients with Parkinson’s disease? Intern. Med. 2013, 52, 1787–1792. [Google Scholar] [CrossRef]
- Nagoor Meeran, M.F.; Jagadeesh, G.S.; Selvaraj, P. Thymol, a dietary monoterpene phenol abrogates mitochondrial dysfunction in β-adrenergic agonist induced myocardial infarcted rats by inhibiting oxidative stress. Chem. Biol. Interact. 2016, 244, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Nagoor Meeran, M.F.; Jagadeesh, G.S.; Selvaraj, P. Thymol attenuates inflammation in isoproterenol induced myocardial infarcted rats by inhibiting the release of lysosome enzymes and downregulating the expressions of proinflammatory cytokines. Eur. J. Pharmacol. 2015, 753, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Riella, K.R.; Marinho, R.R.; Santos, J.S.; Pereira-Filho, R.N.; Cardoso, J.C.; Albuquerque-Junior, R.L.; Thomazzi, S.M. Anti-inflammatory and cicatrizing activities of thymol, a monoterpene of the essential oil from Lippia gracilis, in rodents. J. Ethnopharmacol. 2012, 143, 656–663. [Google Scholar] [CrossRef]
- Boyko, A.A.; Troyanova, N.I.; Kovalenko, E.I.; Sapozhnikov, A.M. Similarity and Differences in Inflammation-Related Characteristics of the Peripheral Immune System of Patients with Parkinson’s and Alzheimer’s Diseases. Int. J. Mol. Sci. 2017, 18, 2633. [Google Scholar] [CrossRef]
- Hunot, S.; Brugg, B.; Ricard, D.; Michel, P.P.; Muriel, M.P.; Ruberg, M.; Faucheux, B.A.; Agid, Y.; Hirsch, E.C. Nuclear translocation of NF-kappaB is increased in dopaminergic neurons of patients with parkinson disease. Proc. Natl. Acad. Sci. USA 1997, 94, 7531–7536. [Google Scholar] [CrossRef]
- Hastings, T.G. Enzymatic oxidation of dopamine: The role of prostaglandin H synthase. J. Neurochem. 1995, 64, 919–924. [Google Scholar] [CrossRef] [PubMed]
- Teismann, P.; Tieu, K.; Choi, D.K.; Wu, D.C.; Naini, A.; Hunot, S.; Vila, M.; Jackson-Lewis, V.; Przedborski, S. Cyclooxygenase-2 is instrumental in Parkinson’s disease neurodegeneration. Proc. Natl. Acad. Sci. USA 2003, 100, 5473–5478. [Google Scholar] [CrossRef]
- Bal-Price, A.; Brown, G.C. Inflammatory neurodegeneration mediated by nitric oxide from activated glia-inhibiting neuronal respiration, causing glutamate release and excitotoxicity. J. Neurosci. 2001, 21, 6480–6491. [Google Scholar] [CrossRef]
- Eve, D.J.; Nisbet, A.P.; Kingsbury, A.E.; Hewson, E.L.; Daniel, S.E.; Lees, A.J.; Marsden, C.D.; Foster, O.J. Basal ganglia neuronal nitric oxide synthase mRNA expression in Parkinson’s disease. Brain Res. Mol. Brain Res. 1998, 63, 62–71. [Google Scholar] [CrossRef]
- Aliabadi, A.; Izadi, M.; Rezvani, M.E.; Esmaeili-Dehaj, M. Effects of thymol on serum biochemical and antioxidant indices in kindled rats. Int. J. Med. Lab. 2016, 3, 43–49. [Google Scholar]
- Mottawie, H.; Ibrahiem, A.; Amer, H. Role of some phytochemicals in the treatment of bronchial asthma. Med. J. Cairo Univ. 2011, 79, 75–80. [Google Scholar]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Javed, H.; Azimullah, S.; Meeran, M.N.; Ansari, S.A.; Ojha, S. Neuroprotective Effects of Thymol, a Dietary Monoterpene Against Dopaminergic Neurodegeneration in Rotenone-Induced Rat Model of Parkinson’s Disease. Int. J. Mol. Sci. 2019, 20, 1538. https://doi.org/10.3390/ijms20071538
Javed H, Azimullah S, Meeran MN, Ansari SA, Ojha S. Neuroprotective Effects of Thymol, a Dietary Monoterpene Against Dopaminergic Neurodegeneration in Rotenone-Induced Rat Model of Parkinson’s Disease. International Journal of Molecular Sciences. 2019; 20(7):1538. https://doi.org/10.3390/ijms20071538
Chicago/Turabian StyleJaved, Hayate, Sheikh Azimullah, MF Nagoor Meeran, Suraiya A Ansari, and Shreesh Ojha. 2019. "Neuroprotective Effects of Thymol, a Dietary Monoterpene Against Dopaminergic Neurodegeneration in Rotenone-Induced Rat Model of Parkinson’s Disease" International Journal of Molecular Sciences 20, no. 7: 1538. https://doi.org/10.3390/ijms20071538
APA StyleJaved, H., Azimullah, S., Meeran, M. N., Ansari, S. A., & Ojha, S. (2019). Neuroprotective Effects of Thymol, a Dietary Monoterpene Against Dopaminergic Neurodegeneration in Rotenone-Induced Rat Model of Parkinson’s Disease. International Journal of Molecular Sciences, 20(7), 1538. https://doi.org/10.3390/ijms20071538