Calcium Activity Dynamics Correlate with Neuronal Phenotype at a Single Cell Level and in a Threshold-Dependent Manner
Abstract
:1. Introduction
2. Results
2.1. Overview of Experimental Plan
2.2. The Intensity of Gene Expression for gad1.1 and slc17a7 is not Directly Correlated with the Metrics of Calcium Activity on a Single-Cell Level
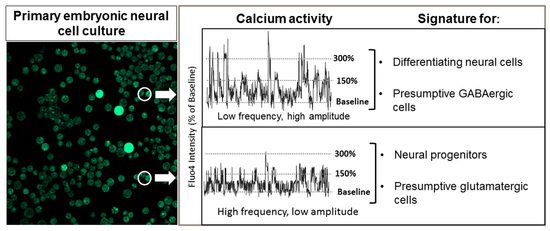
2.3. At Neural Plate Stages, gad1.1-negative Cells Show more Entropic Low-amplitude Calcium Activity while gad1.1 Positive Cells Correlate with more Regular, Higher-Amplitude Activity
2.4. Slc17a7-positive Cells Exhibit Higher Levels of Low-amplitude Spiking than gad1.1-positive Cells, whereas gad1.1-positive Cells Exhibit Higher Levels of high-amplitude Spiking than slc17a7-positive Cells
2.5. The Intensity of Gene Expression for sox2 and tubb2b is not Directly Correlated with the Level of Calcium Activity
2.6. Cells that are Positive for tubb2b or sox2 Show Different Patterns of Calcium Activity than Cells that are Negative for these Markers
2.7. Neural Progenitors and Differentiated Cells Exhibit Significantly Different Patterns of Calcium Activity
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Calcium Imaging
4.2. Calcium Imaging Analysis
4.3. Fluorescence In situ Hybridization (FISH)
4.4. Calculation of FISH Score
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Markova, O.; Sénatore, S.; Chardès, C.; Lenne, P.-F.F. Calcium Spikes in Epithelium: Study on Drosophila Early Embryos. Sci. Rep. 2015, 5, 11379. [Google Scholar] [CrossRef] [PubMed]
- Vidavsky, N.; Shpigel, M.; Addadi, S.; Schertel, A.; Ben-Ezra, D.; Addadi, L.; Weiner, S. Calcium Transport into the Cells of the Sea Urchin Larva in Relation to Spicule Formation. Proc. Natl. Acad. Sci. USA 2016, 113, 12637–12642. [Google Scholar] [CrossRef]
- Chen, J.; Xia, L.; Bruchas, M.R.; Solnica-Krezel, L. Imaging Early Embryonic Calcium Activity with GCaMP6s Transgenic Zebrafish. Dev. Biol. 2017, 430, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J.; Lipp, P.; Bootman, M.D. The Versatility and Universality of Calcium Signalling. Nat. Rev. Mol. Cell Biol. 2000, 1, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Clapham, D.E. Calcium Signaling. Cell 2007, 131, 1047–1058. [Google Scholar] [CrossRef] [Green Version]
- Carafoli, E.; Krebs, J. Why Calcium? How Calcium Became the Best Communicator. J. Biol. Chem. 2016, 291, 20849–20857. [Google Scholar] [CrossRef]
- Giorgi, C.; Danese, A.; Missiroli, S.; Patergnani, S.; Pinton, P. Calcium Dynamics as a Machine for Decoding Signals. Trends Cell Biol. 2018, 28, 258–273. [Google Scholar] [CrossRef] [PubMed]
- Kaneuchi, T.; Sartain, C.V.; Takeo, S.; Horner, V.L.; Buehner, N.A.; Aigaki, T.; Wolfner, M.F. Calcium Waves Occur as Drosophila Oocytes Activate. Proc. Natl. Acad. Sci. USA 2015, 112, 791–796. [Google Scholar] [CrossRef]
- Carvacho, I.; Piesche, M.; Maier, T. Ion Channel Function During Oocyte Maturation and Fertilization. Front. Cell Dev. Biol. 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Liu, Y.; Larsen, K.; Hou, Y.P.; Callesen, H. Calcium-Sensing Receptor (CASR) Is Involved in Porcine in Vitro Fertilisation and Early Embryo Development. Reprod. Fertil. Dev. 2018, 30, 391–398. [Google Scholar] [CrossRef]
- Hao, B.; Webb, S.E.; Miller, A.L.; Yue, J. The Role of Ca2+ Signaling on the Self-Renewal and Neural Differentiation of Embryonic Stem Cells (ESCs). Cell Calcium 2016, 59, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Humeau, J.; Bravo-San Pedro, J.M.; Vitale, I.; Nuñez, L.; Villalobos, C.; Kroemer, G.; Senovilla, L. Calcium Signaling and Cell Cycle: Progression or Death. Cell Calcium 2018, 70, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Tyser, R.C.V.; Miranda, A.M.A.; Chen, C.M.; Davidson, S.M.; Srinivas, S.; Riley, P.R. Calcium Handling Precedes Cardiac Differentiation to Initiate the First Heartbeat. Elife 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Yamamoto, T.S.; Ueno, N. Intracellular Calcium Signal at the Leading Edge Regulates Mesodermal Sheet Migration during Xenopus Gastrulation. Sci. Rep. 2018, 8, 2433. [Google Scholar] [CrossRef]
- Fontana, J.M.; Khodus, G.R.; Unnersjö, D.; Unnersjö-Jess, U.; Blom, H.; Aperia, A.; Brismar, H. Spontaneous Calcium Activity in Metanephric Mesenchymal Cells Regulates Branching Morphogenesis in the Embryonic Kidney. FASEB J. 2018, 33, 4089–4096. [Google Scholar] [CrossRef]
- Bootman, M.D.; Chehab, T.; Bultynck, G.; Parys, J.B.; Rietdorf, K. The Regulation of Autophagy by Calcium Signals: Do We Have a Consensus? Cell Calcium 2018, 70, 32–46. [Google Scholar] [CrossRef]
- Martin, N.; Bernard, D. Calcium Signaling and Cellular Senescence. Cell Calcium. 2018, 70, 16–23. [Google Scholar] [CrossRef]
- Rosenberg, S.S.; Spitzer, N.C. Calcium Signaling in Neuronal Development. Cold Spring Harb. Perspect. Biol. 2011, 3, a004259. [Google Scholar] [CrossRef]
- Brini, M.; Calì, T.; Ottolini, D.; Carafoli, E. Neuronal Calcium Signaling: Function and Dysfunction. Cell. Mol. Life Sci. 2014, 71, 2787–2814. [Google Scholar] [CrossRef]
- Grienberger, C.; Konnerth, A. Imaging Calcium in Neurons. Neuron 2012, 73, 862–885. [Google Scholar] [CrossRef] [Green Version]
- Pachitariu, M.; Stringer, C.; Kenneth, X.; Harris, D. Robustness of Spike Deconvolution for Neuronal Calcium Imaging. J. Neurosci. 2018, 38, 7976–7985. [Google Scholar] [CrossRef]
- Moreau, M.; Néant, I.; Webb, S.E.; Miller, A.L.; Riou, J.F.; Leclerc, C. Ca2+coding and Decoding Strategies for the Specification of Neural and Renal Precursor Cells during Development. Cell Calcium. 2016, 59, 75–83. [Google Scholar] [CrossRef]
- Toth, A.B.; Shum, A.K.; Prakriya, M. Regulation of Neurogenesis by Calcium Signaling. Cell Calcium. 2016, 59, 124–134. [Google Scholar] [CrossRef]
- Borodinsky, L.N. Xenopus laevis as a Model Organism for the Study of Spinal Cord Formation, Development, Function and Regeneration. Front. Neural Circuits 2017, 11, 90. [Google Scholar] [CrossRef]
- Spitzer, N.C. Neurotransmitter Switching in the Developing and Adult Brain. Annu. Rev. Neurosci. 2017, 40. [Google Scholar] [CrossRef]
- Sequerra, E.B.; Levin, J.B.; Castro, P.A.; Borodinsky, L.N.; Goyal, R. NMDA Receptor Signaling Is Important for Neural Tube Formation and for Preventing Antiepileptic Drug-Induced Neural Tube Defects. J. Neurosci. 2018, 38, 4762–4773. [Google Scholar] [CrossRef]
- Lautermilch, N.J.; Spitzer, N.C. Regulation of Calcineurin by Growth Cone Calcium Waves Controls Neurite Extension. J. Neurosci. 2000, 20, 315–325. [Google Scholar] [CrossRef]
- Borodinsky, L.N.; Root, C.M.; Cronin, J.A.; Sann, S.B.; Gu, X.; Spitzer, N.C. Activity-Dependent Homeostatic Specification of Transmitter Expression in Embryonic Neurons. Nature 2004, 429, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.W.; Spitzer, N.C. Spontaneous Calcium Spike Activity in Embryonic Spinal Neurons Is Regulated by Developmental Expression of the Na+, K+-ATPase Beta3 Subunit. J. Neurosci. 2009, 29, 7877–7885. [Google Scholar] [CrossRef]
- Leclerc, C.; Néant, I.; Moreau, M. Early Neural Development in Vertebrates Is Also a Matter of Calcium. Biochimie 2011, 93, 2102–2111. [Google Scholar] [CrossRef] [PubMed]
- Leclerc, C.; Néant, I.; Moreau, M. The Calcium: An Early Signal That Initiates the Formation of the Nervous System during Embryogenesis. Front. Mol. Neurosci. 2012, 5, 1–12. [Google Scholar] [CrossRef]
- Smedler, E.; Uhlén, P. Frequency Decoding of Calcium Oscillations. Biochim. Biophys. Acta Gen. Subj. 2014, 1840, 964–969. [Google Scholar] [CrossRef]
- Paudel, S.; Sindelar, R.; Saha, M. Calcium Signaling in Vertebrate Development and Its Role in Disease. Int. J. Mol. Sci. 2018, 19, 3390. [Google Scholar] [CrossRef]
- Gu, X.; Olson, E.C.; Spitzer, N.C. Spontaneous Neuronal Calcium Spikes and Waves during Early Differentiation. J. Neurosci. Off. J. Soc. Neurosci. 1994, 14, 6325–6335. [Google Scholar] [CrossRef]
- Ciccolini, F.; Collins, T.J.; Sudhoelter, J.; Lipp, P.; Berridge, M.J.; Bootman, M.D. Local and Global Spontaneous Calcium Events Regulate Neurite Outgrowth and Onset of GABAergic Phenotype during Neural Precursor Differentiation. J. Neurosci. 2003, 23, 103–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kramer, I.M. Intracellular Calcium. In Signal. Transtuction, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 381–429. [Google Scholar]
- Uhlén, P. Spectral Analysis of Calcium Oscillations. Sci. Signal. 2004. [Google Scholar] [CrossRef]
- Velazquez-Ulloa, N.A.; Spitzer, N.C.; Dulcis, D. Contexts for Dopamine Specification by Calcium Spike Activity in the CNS. J. Neurosci. 2011, 31, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Guemez-Gamboa, A.; Xu, L.; Meng, D.; Spitzer, N.C. Non-Cell-Autonomous Mechanism of Activity-Dependent Neurotransmitter Switching. Neuron 2014, 82, 1004–1016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marken, J.P.; Halleran, A.D.; Rahman, A.; Odorizzi, L.; LeFew, M.C.; Golino, C.A.; Kemper, P.; Saha, M.S.; Hennings, H.; Michael, D.; et al. A Markovian Entropy Measure for the Analysis of Calcium Activity Time Series. PLoS ONE 2016, 11, e0168342. [Google Scholar] [CrossRef]
- Belgacem, Y.H.; Borodinsky, L.N. Sonic Hedgehog Signaling Is Decoded by Calcium Spike Activity in the Developing Spinal Cord. Proc. Natl. Acad. Sci. USA 2011, 108, 4482–4487. [Google Scholar] [CrossRef]
- Sive, H.L.; Grainger, R.; Harland, R.M. Early Development of Xenopus Laevis: A Laboratory Manual; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2010. [Google Scholar]
- Yao, J.; Pilko, A.; Wollman, R. Distinct Cellular States Determine Calcium Signaling Response. Mol. Syst. Biol. 2016, 12, 894. [Google Scholar] [CrossRef] [PubMed]
- Domenichini, F.; Terrié, E.; Arnault, P.; Harnois, T.; Magaud, C.; Bois, P.; Constantin, B.; Coronas, V. Store-Operated Calcium Entries Control Neural Stem Cell Self-Renewal in the Adult Brain Subventricular Zone. Stem Cells 2018, 36, 761–774. [Google Scholar] [CrossRef]
- Hüser, L.; Novak, D.; Umansky, V.; Altevogt, P.; Utikal, J. Targeting SOX2 in Anticancer Therapy. Expert Opin. Ther. Targets 2018, 22, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Xu, L.; Spitzer, N.C. Target-Dependent Regulation of Neurotransmitter Specification and Embryonic Neuronal Calcium Spike Activity. J. Neurosci. 2010, 30, 5792–5801. [Google Scholar] [CrossRef]
- Pnevmatikakis, E.A.; Merel, J.; Pakman, A.; Paninski, L. Bayesian Spike Inference from Calcium Imaging Data. arXiv, 2013; arXiv:1311.6864. [Google Scholar]
- Theis, L.; Berens, P.; Froudarakis, E.; Reimer, J.; Roman-Roson, M.; Baden, T.; Euler, T.; Tolias, A.; Bethge, M. Supervised Learning Sets Benchmark for Robust Spike Detection from Calcium Imaging Signals. arXiv, 2015; arXiv:1503.00135. [Google Scholar]
- Theis, L.; Berens, P.; Froudarakis, E.; Reimer, J.; Román Rosón, M.; Baden, T.; Euler, T.; Tolias, A.S.; Bethge, M. Benchmarking Spike Rate Inference in Population Calcium Imaging. Neuron 2016, 90, 471–482. [Google Scholar] [CrossRef]
- Zahradníková, A.; Poláková, E.; Zahradník, I.; Zahradníková, A. Kinetics of Calcium Spikes in Rat Cardiac Myocytes. J. Physiol. 2007, 578, 677–691. [Google Scholar] [CrossRef]
- Malmersjö, S.; Rebellato, P.; Smedler, E.; Planert, H.; Kanatani, S.; Liste, I.; Nanou, E.; Sunner, H.; Abdelhady, S.; Zhang, S.; et al. Neural Progenitors Organize in Small-World Networks to Promote Cell Proliferation. Proc. Natl. Acad. Sci. USA 2013, 110, E1524–E1532. [Google Scholar] [CrossRef]
- Tibau, E.; Valencia, M.; Soriano, J. Identification of Neuronal Network Properties from the Spectral Analysis of Calcium Imaging Signals in Neuronal Cultures. Front. Neural Circuits 2013, 7, 199. [Google Scholar] [CrossRef]
- Weissman, T.A.; Riquelme, P.A.; Ivic, L.; Flint, A.C.; Kriegstein, A.R. Calcium Waves Propagate through Radial Glial Cells and Modulate Proliferation in the Developing Neocortex. Neuron 2004, 43, 647–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, H.-H.; Bell, E.; Bates, T.J.D.; Snetkov, V.; Price, J.; Perfect, L.W.; Noristani, H.; Sun, Y.-M.; Uwanogho, D. Neuronatin Promotes Neural Lineage in ESCs via Ca2+ Signaling. Stem Cells 2010, 28, 1950–1960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdul-Wajid, S.; Morales-Diaz, H.; Khairallah, S.M.; Smith, W.C. T-Type Calcium Channel Regulation of Neural Tube Closure and EphrinA/EPHA Expression. Cell Rep. 2015, 13, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Kajikawa, Y.; Hackett, T.A. Entropy Analysis of Neuronal Spike Train Synchrony. J. Neurosci. Methods 2005, 149. [Google Scholar] [CrossRef] [PubMed]
- Fanelli, A.; Esposti, F.; Ion Titapiccolo, J.; Signorini, M.G. Entropy: A Way to Quantify Complexity in Calcium Dynamics. In Proceedings of the XII Mediterranean Conference on Medical and Biological Engineering and Computing, Chalkidiki, Greece, 27–30 May 2010; Springer: Berlin/Heidelberg, Germany, 2010; Volume 29, pp. 343–346. [Google Scholar] [CrossRef]
- Nieuwkoop, P.D.; Faber, J. (Eds.) Normal Table of Xenopus laevis (Daudin): A Systematical and Chronological Survey of the Development from the Fertilized Egg till the End of Metamorphosis; Garland Pub: New York, NY, USA, 1994. [Google Scholar]
- Eilers, P.H.C.; Boelens, H.F.M. Baseline Correction with Asymmetric Least Squares Smoothing. Leiden Univ. Med. Cent. Rep. 2005. [Google Scholar] [CrossRef]
- Qian, B.; Rasheed, K. Hurst Exponent and Financial Market Predictability. In Proceedings of the 2nd IASTED International Conference on Financial Engineering and Applications, Cambridge, MA, USA, 8–10 November 2004; pp. 203–209. [Google Scholar]
- Gleason, K.K.; Dondeti, V.R.; Hsia, H.-L.; Cochran, E.R.; Gumulak-Smith, J.; Saha, M.S. The Vesicular Glutamate Transporter 1 (XVGlut1) Is Expressed in Discrete Regions of the Developing Xenopus Laevis Nervous System. Gene Expr. Patterns 2003, 3, 503–507. [Google Scholar] [CrossRef]
- Watt, S.D.; Gu, X.; Smith, R.D.; Spitzer, N.C. Specific Frequencies of Spontaneous Ca2+ Transients Upregulate GAD 67 Transcripts in Embryonic Spinal Neurons. Mol. Cell. Neurosci. 2000, 16, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Mizuseki, K.; Kishi, M.; Matsui, M.; Nakanishi, S.; Sasai, Y. Xenopus Zic-Related-1 and Sox-2, Two Factors Induced by Chordin, Have Distinct Activities in the Initiation of Neural Induction. Development 1998, 125, 579–587. [Google Scholar]
- Klein, S.L.; Strausberg, R.L.; Wagner, L.; Pontius, J.; Clifton, S.W.; Richardson, P. Genetic and Genomic Tools for Xenopus Research: The NIH Xenopus Initiative. Dev. Dyn. 2002, 225, 384–391. [Google Scholar] [CrossRef]
- Davidson, L.A.; Keller, R.E. Neural Tube Closure in Xenopus Laevis Involves Medial Migration, Directed Protrusive Activity, Cell Intercalation and Convergent Extension. Development 1999, 126, 4547–4556. [Google Scholar]
- Hastie, T. gam: Generalized Additive Models. R Package Version 1.16. Available online: https://cran.r-project.org/package=gam (accessed on 18 March 2019).
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. Available online: https://www.R-project.org/ (accessed on 18 March 2019).





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paudel, S.; Ablondi, E.; Sehdev, M.; Marken, J.; Halleran, A.; Rahman, A.; Kemper, P.; Saha, M.S. Calcium Activity Dynamics Correlate with Neuronal Phenotype at a Single Cell Level and in a Threshold-Dependent Manner. Int. J. Mol. Sci. 2019, 20, 1880. https://doi.org/10.3390/ijms20081880
Paudel S, Ablondi E, Sehdev M, Marken J, Halleran A, Rahman A, Kemper P, Saha MS. Calcium Activity Dynamics Correlate with Neuronal Phenotype at a Single Cell Level and in a Threshold-Dependent Manner. International Journal of Molecular Sciences. 2019; 20(8):1880. https://doi.org/10.3390/ijms20081880
Chicago/Turabian StylePaudel, Sudip, Eileen Ablondi, Morgan Sehdev, John Marken, Andrew Halleran, Atiqur Rahman, Peter Kemper, and Margaret S. Saha. 2019. "Calcium Activity Dynamics Correlate with Neuronal Phenotype at a Single Cell Level and in a Threshold-Dependent Manner" International Journal of Molecular Sciences 20, no. 8: 1880. https://doi.org/10.3390/ijms20081880
APA StylePaudel, S., Ablondi, E., Sehdev, M., Marken, J., Halleran, A., Rahman, A., Kemper, P., & Saha, M. S. (2019). Calcium Activity Dynamics Correlate with Neuronal Phenotype at a Single Cell Level and in a Threshold-Dependent Manner. International Journal of Molecular Sciences, 20(8), 1880. https://doi.org/10.3390/ijms20081880