The Water Extract of Juniperus communis L. Induces Cell Death and Sensitizes Cancer Cells to Cytostatic Drugs through p53 and PI3K/Akt Pathways
Abstract
:1. Introduction
2. Results
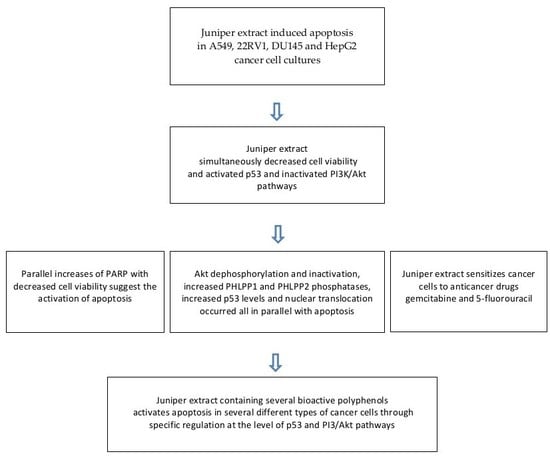
2.1. Juniper Extract Decreased Cell Viability in Several Cancer Cell Lines
2.2. Juniper Extract Activated the p53 Pathway in Parallel with the Inhibition of Cell Proliferation
2.3. Juniper Extract Inactivated the Akt Pathway
2.4. Effects of Juniper Extract on Prostate and Hepatocellular Carcinoma Cells
2.5. Juniper Extract Sensitized A549 Small Lung Cancer Cells to 5-Fluorouracil and Gemcitabine
3. Discussion
4. Materials and Methods
4.1. Plant Material and Reagents
4.2. Cell Culture
4.3. Western Blotting
4.4. Confocal Microscopy
4.5. MTT Assay
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| A549 | Non-small lung cancer cell line |
| DMEM | Dulbecco’s modified Eagle medium |
| DMSO | Dimethyl sulfoxide |
| DU145 | Prostate cancer cell line |
| HepG2 | Hepatocellular carcinoma cell line |
| MTT | 3-(4,5-Dimethylthiazol-2yl)-2,5-diphenyltetrazolium bromide |
| P53 | Transcription factor |
| pAkt | Phosphorylated Akt |
| PARP | Poly (ADP-ribose) polymerase |
| PBS | Phosphate-buffered saline |
| pGsk3Ser9 | Phosphorylated glycogen synthase kinase-3 |
| PHLPP1/2 | Phosphatases |
| PI3K | Phosphatidylinositol-3-kinase |
| PIP3 | Phosphatidylinositol 3,4,5-trisphosphate |
| TEA | Triethanolamine-HCl |
References
- Lantto, T.A.; Colucci, M.; Závadová, V.; Hiltunen, R.; Raasmaja, A. Cytotoxicity of curcumin, resveratrol and plant extracts from basil, juniper, laurel and parsley in SH-SY5Y and CV1-P cells. Food Chem. 2009, 117, 405–411. [Google Scholar] [CrossRef]
- Lantto, T.A.; Dorman, H.J.D.; Shikov, A.N.; Pozharitskaya, O.N.; Makarov, V.G.; Tikhonov, V.P.; Hiltunen, R.; Raasmaja, A. Chemical composition, antioxidative activity and cell viability effects of a Siberian pine (Pinus sibirica Du Tour) extract. Food Chem. 2009, 112, 936–943. [Google Scholar] [CrossRef]
- Lantto, T.A.; Laakso, I.; Dorman, H.J.; Mauriala, T.; Hiltunen, R.; Kõks, S.; Raasmaja, A. Cellular Stress and p53-Associated Apoptosis by Juniperus communis L. Berry Extract Treatment in the Human SH-SY5Y Neuroblastoma Cells. Int. J. Mol. Sci. 2016, 17, 1113. [Google Scholar] [CrossRef] [PubMed]
- Iurlaro, R.; León-Annicchiarico, C.L.; Muñoz-Pinedo, C. Regulation of Cancer Metabolism by Oncogenes and Tumor Suppressors. Meth. Enzymol. 2014, 542, 59–80. [Google Scholar] [PubMed]
- Whibley, C.; Pharoah, P.D.P.; Hollstein, M. p53 polymorphisms: Cancer implications. Nat. Rev. Cancer 2009, 9, 95–107. [Google Scholar] [CrossRef]
- Bieging, K.T.; Mello, S.S.; Attardi, L.D. Unravelling mechanisms of p53-mediated tumour suppression. Nat. Rev. Cancer 2014, 14, 359–370. [Google Scholar] [CrossRef] [Green Version]
- Rodgers, S.J.; Ferguson, D.; Mitchell, C.; Ooms, L. Regulation of PI3K effector signalling in cancer by the phosphoinositide phosphatases. Biosci. Rep. 2017, 12, BSR20160432. [Google Scholar] [CrossRef]
- O’Neill, A.K.; Niederst, M.J.; Newton, A.C. Suppression of survival signalling pathways by the phosphatase PHLPP. FEBS J. 2013, 280, 572–583. [Google Scholar] [CrossRef]
- Tiligada, E. Nuclear translocation during the cross-talk between cellular stress, cell cycle and anticancer agents. Curr. Med. Chem. 2006, 13, 1317–1320. [Google Scholar] [CrossRef] [PubMed]
- Grzechnik, A.T.; Newton, A.C. PHLPPing through history: A decade in the life of PHLPP phosphatases. Biochem. Soc. Trans. 2016, 44, 1675–1682. [Google Scholar] [CrossRef]
- O’Brate, A.; Giannakakou, P. The importance of p53 location: Nuclear or cytoplasmic zip code? Drug Resist. Updates 2003, 6, 313–322. [Google Scholar] [CrossRef]
- Roudier, E.; Mistafa, O.; Stenius, U. Statins induce mammalian target of rapamycin (mTOR) mediated inhibition of Akt signaling and sensitize p53-deficient cells to cytostatic drugs. Mol. Canc. Ther. 2006, 5. [Google Scholar] [CrossRef]
- Mistafa, O.; Stenius, U. Statins inhibit Akt/PKB signaling via P2X7 receptor in pancreatic cancer cells. Biochem. Pharmacol. 2009, 78, 1115–1126. [Google Scholar] [CrossRef] [Green Version]
- Miraglia, E.; Högberg, J.; Stenius, U. Statins exhibit anticancer effects through modifications of the pAkt signaling pathway. Int. J. Oncol. 2012, 40, 867–875. [Google Scholar] [CrossRef]
- Barron, C.C.; Moore, J.; Tsakiridis, T.; Pickering, G.; Tsiani, E. Inhibition of human lung cancer cell proliferation and survival by wine. Cancer Cell Internat. 2014, 14, 6. [Google Scholar] [CrossRef]
- Moore, J.; Megaly, M.; MacNeil, A.J.; Klentrou, P.; Tsiani, E. Rosemary extract reduces Akt/mTOR/p70S6K activation and inhibits proliferation and survival of A549 human lung cancer cells. Biomed. Pharmacother. 2016, 83, 725–732. [Google Scholar] [CrossRef]
- Hinneburg, I.; Dorman, H.J.D.; Hiltunen, R. Antioxidant activities of extracts from selected culinary herbs and spices. Food Chem. 2006, 97, 122–129. [Google Scholar] [CrossRef]
- Das, A.; Banik, N.L.; Ray, S.K. Mechanism of apoptosis with the involvement of calpain and caspase cascades in human malignant neuroblastoma SH-SY5Y cells exposed to flavonoids. Int. J. Cancer 2006, 119, 2575–2585. [Google Scholar] [CrossRef] [Green Version]
- Karmakar, S.; Davis, K.A.; Choudhury, S.R.; Deeconda, A.; Banik, N.L.; Ray, S.K. Bcl-2 inhibitor and apigenin worked synergistically in human malignant neuroblastoma cell lines and increased apoptosis with activation of extrinsic and intrinsic pathways. Biochem. Biophys. Res. Commun. 2009, 388, 705–710. [Google Scholar] [CrossRef] [Green Version]
- Lee, E.R.; Kang, Y.J.; Choi, H.Y.; Kang, G.H.; Kim, J.H.; Kim, B.W.; Han, Y.S.; Nah, S.Y.; Paik, H.D.; Park, S.Y.; et al. Induction of Apoptotic Cell Death by Synthetic Naringenin Derivatives in Human Lung Epithelial Carcinoma A549 Cells. Biol. Pharm. Bull. 2007, 30, 2394–2398. [Google Scholar] [CrossRef] [Green Version]
- Xingyu, Z.; Peijie, M.; Dan, P.; Youg, W.; Daojun, W.; Xinzheng, C.; Xijun, Z.; Yangrong, S. Quercetin suppresses lung cancer growth by targeting Aurora B kinase. Canc. Med. 2016, 5, 3156–3165. [Google Scholar] [CrossRef] [Green Version]
- Lehár, J.; Krueger, A.S.; Avery, W.; Heilbut, A.M.; Johansen, L.M.; Price, E.R.; Rickles, R.J.; Short, G.F.; Staunton, J.E.; Jin, X.; et al. Synergistic drug combinations tend to improve therapeutically relevant selectivity. Nat. Biotechnol. 2009, 27, 659–666. [Google Scholar] [CrossRef] [Green Version]
- Tsai, J.Y.; Tsai, S.H.; Wu, C.C. The chemopreventive isothiocyanate sulforaphane reduces anoikis resistance and anchorage-independent growth in non-small cell human lung cancer cells. Toxicol. Appl. Pharmacol. 2019, 362, 116–124. [Google Scholar] [CrossRef]
- Guntur, V.P.; Waldrep, J.C.; Guo, J.J.; Selting, K.; Dhand, R. Increasing P53 Protein Sensitizes Non-Small Cell Lung Cancer to Paclitaxel and Cisplatin In Vitro. Anticancer Res. 2010, 30, 3557–3564. [Google Scholar]
- Gorzynik-Debicka, M.; Przychodzen, P.; Cappello, F.; Kuban-Jankowska, A.; Gammazza, A.M.; Knap, N.; Wozniak, M.; Gorska-Ponikowska, M. Potential Health Benefits of Olive Oil and Plant Polyphenols. Int. J. Mol. Sci. 2018, 19, 547. [Google Scholar] [CrossRef]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raasmaja, A.; Stenius, U.; Ghalali, A. The Water Extract of Juniperus communis L. Induces Cell Death and Sensitizes Cancer Cells to Cytostatic Drugs through p53 and PI3K/Akt Pathways. Int. J. Mol. Sci. 2019, 20, 2054. https://doi.org/10.3390/ijms20092054
Raasmaja A, Stenius U, Ghalali A. The Water Extract of Juniperus communis L. Induces Cell Death and Sensitizes Cancer Cells to Cytostatic Drugs through p53 and PI3K/Akt Pathways. International Journal of Molecular Sciences. 2019; 20(9):2054. https://doi.org/10.3390/ijms20092054
Chicago/Turabian StyleRaasmaja, Atso, Ulla Stenius, and Aram Ghalali. 2019. "The Water Extract of Juniperus communis L. Induces Cell Death and Sensitizes Cancer Cells to Cytostatic Drugs through p53 and PI3K/Akt Pathways" International Journal of Molecular Sciences 20, no. 9: 2054. https://doi.org/10.3390/ijms20092054
APA StyleRaasmaja, A., Stenius, U., & Ghalali, A. (2019). The Water Extract of Juniperus communis L. Induces Cell Death and Sensitizes Cancer Cells to Cytostatic Drugs through p53 and PI3K/Akt Pathways. International Journal of Molecular Sciences, 20(9), 2054. https://doi.org/10.3390/ijms20092054