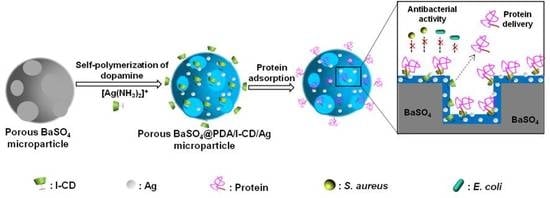
1. Introduction
Poly(methyl methacrylate) (PMMA)-based bone cements have been widely used in orthopaedic open surgeries such as artificial joint replacement and minimally invasive surgeries such as vertebroplasty and kyphoplasty for treating vertebral fractures [
1,
2,
3]. Although PMMA has excellent mechanical properties, it is not radiopaque. Therefore, a radiopaque agent is commonly needed in PMMA bone cements. Various radiopaque agents, including barium sulfate (BaSO
4), zirconium dioxide (ZrO
2), tungsten, tantalum, and iodinated methacrylate, have been used in PMMA bone cements [
4,
5,
6,
7,
8,
9,
10]. Because of its excellent stability and outstanding optical properties, BaSO
4 has been most commonly used as radiopaque agent in PMMA bone cements [
9,
11,
12,
13].
However, BaSO
4 exists some problems in clinical application and needs to be further improved. As an inorganic filler, the chemical inertness nature of BaSO
4 causes insufficient interaction between BaSO
4 particles and PMMA matrix, adversely affecting the mechanical properties of bone cements [
14]. In addition, BaSO
4 has inherent cytotoxicity, which may lead to the loosening of implants [
15]. Further modification of BaSO
4 may improve its performance in PMMA bone cements. Micro/nano-sized BaSO
4 particles have high specific surface area, and which may be benefit for improving its biocompatibility and optical imaging property [
16]. In recent years, porous materials have been widely used in the field of catalysis, adsorption, and drug delivery due to their large specific surface area, tailored pore size, structure and surface properties [
17]. With the purpose to further increase the specific surface area of BaSO
4 particles to enhance biocompatibility and realize multifunction, porous BaSO
4 particles are raised [
11,
16]. Nandakumar et al. prepared monodispersed spheroid BaSO
4 microparticles with porous structure via direct precipitation of barium chloride (BaCl
2) and ammonium sulfate ((NH
4)
2SO
4) in aqueous polyvinyl alcohol (PVA) solution [
11]. Chen et al. fabricated mesoporous BaSO
4 microspheres with a larger pore size via Ostwald Ripening at room temperature, which will act as promising candidates for catalyst carrier, adsorbents, and so on [
16].
A PDA-assisted modification method is appropriate for further modification of porous BaSO
4 particles. The development of multifunctional radiopaque agent has become a trend. Hence, except X-ray absorption ability, the new radiopaque agent is expected to have antibacterial activity and therapeutic effect. Previous studies reported many surface modification techniques, among them PDA-assisted modification method is popular [
18,
19]. Dopamine molecules can undergo self-polymerization and form extraordinary adhesion on a wide variety of substrate surfaces under weak alkaline conditions [
20]. More importantly, PDA coating can act as anchors to graft the secondary functional biopolymers with thiols and amines via Michael addition or Schiff base reactions. In the previous study, PDA was coated on to the surface of polystyrene culture plates for further decorated with liposomes [
20]. Moreover, the catechol groups in PDA coating can also reduce metal ions to elemental metals, generating metallic microparticles on the surface of various materials.
The antibacterial property of BaSO
4 particles could be realized by PDA-assisted Ag deposition. Ag modification is a useful method to inhibit bacterial infection [
17,
21,
22,
23]. Inflammation caused by bacteria is common in patients after surgeries. An important type of antibacterial material, Ag may help improve the antibacterial property of implants and facilitate healing when it is incorporated in the implants. Ag nanoparticles could be introduced to the surface of materials via reduction of Tollens’ reagent by PDA. In a previous study, we successfully modified poly(ether ether ketone) (PEEK) implants using PDA-based surface modification technology and subsequent deposition of Ag nanoparticles on the surface of the implants. This coating exhibited outstanding antibacterial property against both
Staphylococcus aureus (
S. aureus) and
Escherichia Coli (
E. coli) in vitro as well as decent antibacterial performance in vivo [
24]. Therefore, the technique of PDA-assisted deposition of Ag may be also suitable for BaSO
4 particles.
The therapeutic effect of BaSO
4 particles as radiopaque agent could be endowed with loading therapeutic drugs by porous structure, PDA coating and cyclodextrin (CD). The porous BaSO
4 particles can load drugs [
17]. PDA modification may also promote protein adsorption on the surface of particles [
20]. There are different methods to encapsulate drugs, among them I-CD represents excellent drug loading property contributing to inclusion complex formation of CD with a wide array of small molecules [
25,
26,
27,
28]. Rivera-Delgado et al. synthesized different cyclodextrin polymers to control the delivery rates from solid polymer from hours and days, to weeks and months [
28]. In addition, CD can also help to inhibit the aggregation of therapeutic proteins released from the porous particles. Because the CD can incorporate hydrophobic residues on aggregation-prone proteins or on their partially unfolded intermediates into the hydrophobic cavity [
29]. Therefore, I-CD will be introduced into the PDA coating for drug encapsulation. The presence of porous structure, PDA coating and CD could control the delivery and maintain the bioactive of therapeutic protein drugs. In addition, the presence of I may also enhance the X-ray absorption ability.
With the goal to gain a radiopaque agent having strong radiopacity, great antibacterial property and long drug delivery ability, we developed novel porous BaSO
4@PDA/I-CD/Ag microparticles with multifunctional coating (
Scheme 1). As both BaSO
4 and iodo-compounds are common radiopaque agents in radiography and computed tomography (CT), the combination of them may further improve the radiopacity of radiopaque agent [
30]. The PDA coating, polymerized from dopamine in alkaline condition, has been proved to improve the biocompatibility as surface coating outside the microparticles. The PDA coating, I-CD and pores in BaSO
4 microparticles can load drugs for promoting bone formation. Moreover, in situ deposition of Ag on the surface of the PDA coating could be realized, that will endow the material with an antibacterial property. Scanning electron microscope (SEM) and energy-dispersive X-ray spectroscope (EDS) will be used to observe the structure and measure elementary composition of porous BaSO
4@PDA/I-CD/Ag microparticles. The drug release behavior of porous BaSO
4@PDA/I-CD/Ag microparticles is going to be detected. X-ray absorption measurements and mechanical property of PMMA bone cements containing porous BaSO
4@PDA/I-CD/Ag microparticles will also be evaluated. The mechanical property of PMMA bone cements containing particles will be studied. The antibacterial performance of PMMA bone cements added porous BaSO
4@PDA/I-CD/Ag microparticles will be investigated using
S. aureus and
E. coli. The biocompatibility of PMMA bone cement containing porous BaSO
4@PDA/I-CD/Ag microparticles will be determined using MC3T3-E1 cells.
3. Discussion
The aim of this study is to prepare multifunctional radiopaque agent for bone cements. BaSO
4 microparticle, a common radiopaque agent, is used as a substrate material for further modification. According to the SEM results, it can be seen that porous BaSO
4 microparticle has been successfully fabricated. The pores in the particles can make porous BaSO
4 microparticles itself became a good carrier for therapeutic drugs. Similarly, the pores may also be used to increase drug loading for other radiopaque agent. To realize the multifunction, further surface modification of this porous BaSO
4 microparticle is carried out. In this study, PDA coating is used as a platform for modification, which is an easy method for surface modification, is not only applied to modify most materials but also forms functional groups for further reaction [
17,
20,
21,
22]. EDS result shows that Ag and I-CD are modified on the surface of porous BaSO
4 microparticles with the help of PDA coating. Porous BaSO
4 microparticles may absorb some Ag and I-CD to a certain extent because of existed pores, but the PDA coating will improve the stability and amount of Ag and I-CD. Additionally, the PDA coating may also improve the interface strength between PMMA and BaSO
4 particles and be used to absorb drugs.
Because of the porous structure, BSA can be encapsulated into porous BaSO
4 microparticle. Compared to porous BaSO
4, porous BaSO
4@PDA shows higher encapsulation rate. This result may be attributed to protein adsorption of PDA coating. It is well known that CD can load drugs contributing to inclusion complex formation of CD with a wide array of small molecules and stabilize protein bioactivity in the liquid and dried state by inhibiting the protein aggregation [
25,
26,
27,
28,
29], combining the function of PDA adsorption and CD, porous BaSO
4@PDA/I-CD/Ag microparticle exhibits highest drug loading efficiency. Compared with porous BaSO
4, porous BaSO
4@PDA, porous BaSO
4@PDA/I-CD/Ag microparticles exhibited burst release of BSA. The faster release rate of pure particles and cements containing porous BaSO
4@PDA and porous BaSO
4@PDA/I-CD/Ag microparticles than porous BaSO
4 microparticles may be attributed to the improvement of hydrophilicity after PDA coating. Although the release rate of BaSO
4@PDA/I-CD/Ag is faster, the released amount of BSA in this group at each time point is most. The results show that the designed multifunctional coatings on the surface of porous BaSO4 microparticles work as intended. Therefore, the prepared porous BaSO
4@PDA/I-CD/Ag microparticles still have potential application as a drug carrier.
PMMA bone cement shows excellent mechanical property and its drug loading and release property is demand. Utilizing mesoporous silica nanoparticles to load antibiotics can improve anti-infection property of bone cement. The incorporation of MSNs to the bone cements (8.15 wt%) shows no detrimental effects on the biomechanical properties of bone cements, meanwhile sustained drug delivery from bone cement endows cement anti-infection property and promotes its therapeutic. CD can load various drugs, the addition of CD microparticles to cement enables incorporation of previously incompatible antibiotics while preserves favorable mechanical properties [
23,
25,
31]. In this study, the fabricated porous BaSO
4@PDA/I-CD/Ag microparticle in PMMA bone cement can load and release protein. Compared with cement containing porous BaSO
4, samples containing porous BaSO
4@PDA/I-CD/Ag microparticle can release more BSA at the same time due to the multifunctional coating. Therefore, PMMA bone cement containing porous BaSO
4@PDA/I-CD/Ag microparticle may be a potential drug carrier.
PMMA bone cement containing porous BaSO4@PDA/I-CD/Ag microparticles showed good X-ray imaging efficiency. I element has good X-ray absorption property and it is often used as contrast agents in clinical practice. Ag, as a metallic element, can also absorb X-ray. Here, the radiopacity of BaSO4@PDA/Ag and BaSO4@PDA/I-CD are significantly enhanced by the introduction of Ag or I. PMMA bone cements containing above microparticles exhibit excellent X-ray imaging property and porous BaSO4@PDA/I-CD/Ag may be applied as a new type radiocontrast for clinical therapeutics.
It was reported that the addition of additives into PMMA bone cements would decrease the mechanical property [
32,
33]. In our group’s previous study, the compressive strength also decreased with addition of magnesium balls [
33]. This study shows the same results with previous reports. The results demonstrate that the addition of particles will reduce the mechanical properties of PMMA bone cements slightly. Even the amount of porous BaSO
4@PDA/I-CD/Ag microparticles in bone cements reaches 10 wt%, the compressive strength is larger than standard (ISO 5833, 70 MPa). That is contributed to the multifunctional coating on the surface of porous BaSO
4 particle, it can enhance the interaction between particles and PMMA bone cement matrix via functional groups of PDA coating. Therefore, PMMA bone cement can maintain a high compressive strength even if adding a large amount of porous BaSO
4@PDA/I-CD/Ag. The mechanical property of lumbar vertebrae filled with cement containing BaSO
4@PDA/I-CD/Ag microparticles is higher than samples containing commercial BaSO
4 particles, further demonstrating that BaSO
4@PDA/I-CD/Ag microparticles can improve the strength of cement.
Silver-based nanomaterials are known for their strong antibacterial activities for a lot of bacteria [
17,
21,
22]. Previous studies indicated the presence of catechol structure in polydopamine could inhibit the growth and proliferation of bacteria in some extent. However, the antibacterial ability of PDA is far less than the actual clinical needs. In this study, silver is in situ deposited onto the surface of porous BaSO
4@PDA microparticles with the help of PDA-based surface modification technology to gain porous BaSO
4@PDA/Ag or porous BaSO
4@PDA/I-CD/Ag microparticles. As shown, even the additional amount of BaSO
4@PDA/Ag or porous BaSO
4@PDA/I-CD/Ag microparticles is only 7.5 wt%, remarkable antibacterial effect is obtained. The results show that the multifunctional coating on the surface of microparticles is effective in preventing bacterial infection. Therefore, this multifunctional coating may be favorable for radiopaque agent modification.
The biocompatibility of radiopaque agents is another factor affecting the clinical application of bone cements. In this study, 7.5 wt% is thought to be an optimized amount. Then the cytotoxicity of the extracts of PMMA bone cement and PMMA bone cements adding commercial BaSO4, porous BaSO4, porous BaSO4@PDA, porous BaSO4@PDA/Ag and porous BaSO4@PDA/I-CD or porous BaSO4@PDA/I-CD/Ag particles are detected using MC3T3-E1 cells. PDA is a kind of functional polymer coating, which not only does not produce toxicity, but also improves the biocompatibility of substrate materials and promotes cell adhesion. Compared with pure PMMA bone cement, the samples adding different particles show no obvious cytotoxicity. Hence, this multifunctional coating may be a safe approach for radiopaque agent modification.
4. Materials and Methods
4.1. Preparation and Characterizations of Porous BaSO4@PDA/I-CD/Ag Microparticles
Porous BaSO
4 microparticles were prepared according to the literature [
11]. In brief, 5.2 g BaCl
2 (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) was dissolved in 50 mL deionized water and thoroughly mixed with 50 mL 3 wt% PVA (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) solution under vigorous stirring. Then 25 mL deionized water containing 3.3 g (NH
4)
2SO
4 (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) was dropped into the above solution through a constant pressure titration funnel at a rate of two drops per second. After that, the mixture was settled for 5 h at room temperature. The precipitate was thoroughly washed with deionized water to remove redundant PVA, unreacted reagents and by-products and then dried at 80 °C for 2 h to obtain BaSO
4 microparticles. Porous BaSO
4 microparticles were prepared by further calcining the BaSO
4 microparticles at 600 °C for 4 h.
To prepare porous BaSO4@PDA microparticles, 3 g porous BaSO4 microparticles were dispersed in a dopamine (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) solution, which was prepared by dissolving 300 mg dopamine hydrochloride (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) and 120 mg copper sulfate (CuSO4) (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) in 150 mL Tris solution (50 mM, pH = 8.5). After 111 μL of hydrogen peroxide (H2O2) (Chengdu area of the ndustrial development zone xindu mulan, Chengdu, China) was added, the mixture was stirred for 40 min at 25 °C. Further, porous BaSO4@PDA/I-CD microparticles were prepared by adding 1 mL N, N-dimethylformamide (DMF) (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) solution containing 100 mg I-CD (Zhiyuan Biotechnology Co., Ltd., Binzhou, China) quickly prior to adding H2O2. To obtain porous BaSO4@PDA/Ag microparticles, 3 g porous BaSO4 microparticles were added into 150 mL Tollens’ solution (0.02 M) and then mixed with 150 mL dopamine solution (2 mg mL−1), followed by stirring at 25 °C for 8 h. Finally, the porous BaSO4@PDA/I-CD/Ag microparticles were fabricated by mixing 150 mL Tollens’ reagent (0.02 M) containing 3 g BaSO4 microparticles, and 150 mL dopamine solution (2 mg mL−1) containing 100 mg I-CD. This mixture was vigorously stirred at 25 °C for 8 h to obtain porous BaSO4@PDA/I-CD/Ag microparticles.
SEM (Quanta 250, FEI, Hillsboro, OR, USA) and TEM (Tecnai G2 F20, OR, USA) were used to characterize the morphology of all microparticles. EDS (INCA X-Max 250, EDAX Inc, PA, USA) was used for surface chemical analysis of microparticles.
4.2. Drug Release Measurements
In order to measure the drug delivery behavior of particles, BSA (Guangzhou Saiguo Biotech Co., Ltd., Guangzhou, China) was used a model drug in this study. In brief, 100 mg porous BaSO4, porous BaSO4@PDA or porous BaSO4@PDA/I-CD/Ag were dispersed in 1 mL BSA solution (2 mg mL−1), and shaken at 37 °C for 2 h. Drug-loaded microparticles were obtained by centrifuging and drying in vacuum oven overnight. For the drug release tests, 20 mg BSA-loaded microparticles were added into 1 mL phosphate buffer saline (PBS) (Hyclone, UT, USA) solution and shaken at 37 °C. At designed time points, 0.25 mL supernatant was sampled and 0.25 mL fresh PBS was added. Three duplicates were analyzed at each time point in this test. The concentration of BSA was analyzed by using a micro BCA protein assay kit (Beijing ComWin Biotech Co., Ltd., Beijing, China) at the wavelength of 562 nm. The release of BSA from PMMA bone cement containing different particles was also investigated and the method to fabricate PMMA bone cement was as followed.
4.3. Preparation of PMMA Bone Cements Containing Microparticles
PMMA bone cements contain powder and liquid, the mass to volume ratio of them is 2:1. The solid includes PMMA (M. W 35,000, Sigma-Aldrich, Shanghai, China), radiopaque agent and 2% (w/w) di-benzoyl peroxide (BPO) (Sigma-Aldrich, Shanghai, China). While the liquid is consisted of 99% methyl methacrylate (MMA) (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) and 1% (v/v) N,N-dimethyl-p-toluidine (DMPT) (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China). Herein, 3 g powder and 1.5 mL liquid were used to fabricate pure PMMA bone cements. In this study, four types of mass ratio about the porous BaSO4@PDA/I-CD/Ag microparticles to solid phase (2.5 wt%, 5 wt%, 7.5 wt%, 10 wt%) were added in PMMA bone cements. The production of PMMA bone cements containing 2.5 wt% porous microparticles is described as an example. The solid powder including 2.925 g PMMA, 0.075 g porous microparticles and 0.006 g BPO were mixed in mortar, 1.5 mL well-mixed liquid including 1.485 mL MMA and 0.015 mL DMPT was added into the mortar. The mixture was removed to molds before solidification. PMMA bone cements containing porous microparticles could be obtained a few minutes later. The bone cements containing commercial BaSO4, porous BaSO4, porous BaSO4@PDA, porous BaSO4@PDA/Ag and porous BaSO4@PDA/I-CD microparticles were prepared with the same method mentioned above.
4.4. Radiopacity Measurements
The cylindrical PMMA bone cements (height: 12 mm, diameter: 6 mm) containing 2.5 wt%, 5 wt%, 7.5 wt% and 10 wt% porous BaSO4@PDA/I-CD/Ag microparticles were measured via micro-CT scanning (SkyScan 1176, SkyScan, Aartselaar, Belgium) for Hu value analysis. Three samples were detected for each group. The radiopacity of the PMMA bone cements formulated with different radiopaque agents in a defect (length: 20 mm, width: 20 mm, height: 10 mm) of isolated vertebral body of sheep was also detected. Before the X-ray imaging, PMMA bone cements (Control group), PMMA bone cements containing 7.5 wt% commercial BaSO4 (Commercial BaSO4 group) and 7.5 wt% porous BaSO4@PDA/I-CD/Ag microparticles (BaSO4@PDA/I-CD/Ag group) were injected in defect and solidified, respectively.
4.5. Mechanical Tests
To evaluate effect of these radiopaque agents on the mechanical property of PMMA bone cements, compression tests were carried out using universal mechanical testing machine (E10000, Instron, Hengyi precision instrument Co., Ltd., Shanghai, China) with three repetitions for each group. In order to investigate the effect of adding an amount of porous BaSO4@PDA/I-CD/Ag microparticles in PMMA bone cements on compressive strength, PMMA bone cements containing 0 wt%, 2.5 wt%, 5 wt%, 7.5 wt% and 10 wt% porous BaSO4@PDA/I-CD/Ag microparticles were prepared. To compare the effect of different microparticles on compressive strength, PMMA bone cements adding 7.5 wt% commercial BaSO4, porous BaSO4, porous BaSO4@PDA, porous BaSO4@PDA/Ag, porous BaSO4@PDA/I-CD and porous BaSO4@PDA/I-CD/Ag microparticles were then fabricated. The cements were hardened in pillar mold with diameter of 6 mm and height of 12 mm for 1 h and then ejected. The rate for these compression tests were 1 mm min−1. Male sheep lumbar vertebrae (L1-L6), decalcified in 10% 10% EDTA Na2 (Solarbio, Beijing Solarbio Science & Technology Co., Ltd., Beijing, China) for 20 days, was used to investigate the mechanical property of PMMA bone cement containing 7.5 wt% of porous BaSO4, and BaSO4@PDA/I-CD/Ag microparticles. The rate for these compression tests were 5 mm min−1.
4.6. Bacteria Morphology Observation
The antibacterial performance of PMMA bone cements containing porous BaSO4, porous BaSO4@PDA, porous BaSO4@PDA/Ag, porous BaSO4@PDA/I-CD and porous BaSO4@PDA/I-CD/Ag microparticles were evaluated using S. aureus and E. coli (supplied by China General Microbiological Culture Collection Center, Beijing, China). Both S. aureus and E. coli were cultured in Luria-Bertani (LB) medium at 37 °C for 24 h. PMMA bone cements were also detected as control group. The bacteria density was adjusted to be concentration of 105 CFU mL−1 in the antibacterial assay. S. aureus and E. coli were seeded on the bone cements disc with diameter 5.8 mm. After 24 h of incubation, the samples were washed with PBS for three times to eliminate non-adherent bacteria. After fixing in 2.5% glutaraldehyde and dehydrating in graded ethanol/ distilled water mixture (50%, 60%, 70%, 80%, 90% and 100%), the samples were then dried using a Critical Point Dryer (CPD030, LEICA, Wetzlar, Germany) and sputter-coated with gold using an Ion Sputter (SC7620, Quorum Technologies, Lewes, UK). Finally, the morphology of bacteria was observed by SEM.
4.7. Antibacterial Efficiency Activity of Cement Containing Particles
The inhibition zone test was employed to evaluate bacterial inhibition activity of particles using a modified disk diffusion method. S. aureus and E. coli were selected as model bacteria for assay. Briefly, the PMMA bone cement discs (diameter: 10 mm; high: 1mm) containing 7.5 wt% commercial BaSO4, BaSO4@PDA/Ag and BaSO4@PDA/Ag particles were fabricated, 100 μL of fresh bacterial suspension (S. aureus and E. coli, 106–107 colony forming units per mL) was spread on LB agar plates. Afterward, the PMMA bone cement discs were gently placed on the center of LB agar plates and cultured at 37 °C for 24 h. The size of the zone around disks was measured to evaluate the bacterial inhibition activity of cement containing particles.
4.8. Antibacterial Efficiency
The bacterial inhibition rate of PMMA bone cements containing porous BaSO
4@PDA/Ag or porous BaSO
4@PDA/I-CD/Ag microparticles compared to PMMA bone cements was studied. Each sample (3 repetitions) was incubated in 10 μL of the bacteria suspension in LB medium at 37 °C for 24 h. Then the samples were gently washed thrice with PBS. Afterwards, the adhered bacteria on each sample were detached into 1 mL of PBS by vortex oscillator for 3 min, and the resulting bacterial suspensions were used to count the viable bacteria adhered on the specimens. The antibacterial rates were calculated based on the following formula:
in which A represents the average number of viable bacteria attached on the PMMA bone cements containing porous BaSO
4@PDA/Ag or porous BaSO
4@PDA/I-CD/Ag microparticles, and B represents the number of bacteria on PMMA bone cements.
4.9. Cytotoxicity
The cytotoxicity is evaluated by culturing MC3T3-E1 cells using the extracts of PPMMA bone cements containing 7.5 wt% of commercial BaSO4, porous BaSO4, porous BaSO4@PDA, porous BaSO4@PDA/Ag, porous BaSO4@PDA/I-CD and porous BaSO4@PDA/I-CD/Ag particles. The amount of culture medium was added according to the standard of 0.2 mL per gram bone cement. All the bone cements were sterilized for cell culture. The cell growth using the normal culture medium (TCPs group) and the extract of pure PMMA bone cements (PMMA group) were also determined. All the cements were immersed into a MEM alpha modification medium for 24 h, and filtered by 0.22 μm sterile filter head. MC3T3-E1 cells were cultured in 96-well plate (5 × 103 cells/well) with 300 μL normal medium or extracts in a 37 °C incubator with 5% CO2. The normal culture medium was prepared by adding 10% (v/v) FBS and 1% (v/v) penicillin/streptomycin (Gibco, ThermoFisher Scientific, CA, USA) in MEM alpha modification medium. After culturing of 1, 2 and 3 days, fresh 100 μL MEM alpha modification medium (Hyclone, UT, USA) and 10 μL CCK-8 agent (Dojindo, Kumamoto, Japan) were added and incubated for 2 h in a 37 °C incubator with 5% CO2. The absorbance at a wavelength of 450 nm was detected.
4.10. The Morphology of MC3T3-E1 Cells on the Surface of Bone Cements
The MC3T3-E1 cells were seeded on the surface of disc of bone cements (diameter: 10 mm, height: 2 mm) in a 24-well plate. For this experiment, pure PMMA bone cement and PMMA bone cements containing commercial BaSO4, porous BaSO4, porous BaSO4@PDA, porous BaSO4@PDA/Ag, porous BaSO4@PDA/I-CD and porous BaSO4@PDA/I-CD/Ag particles were prepared. After culturing for 48 h, the samples were washed three times with PBS, fixed with 4% paraformaldehyde for 40 min, and washed three times with deionized water afterwards. The samples were then dehydrated with gradient ethanol (10%, 30%, 50%, 70%, 70%, 85%, 85%, 90%, 100% and 100%) and dried using a critical point drier. Before SEM observation, all the samples were treated by platinum sputtering for 45 s.
4.11. Statistical Analysis
All experiments were performed in triplicate unless otherwise specified. The data were expressed as the means ± standard deviations. Statistical analysis was performed using one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test using SPSS software. Differences at p < 0.05 were considered statistically significant.