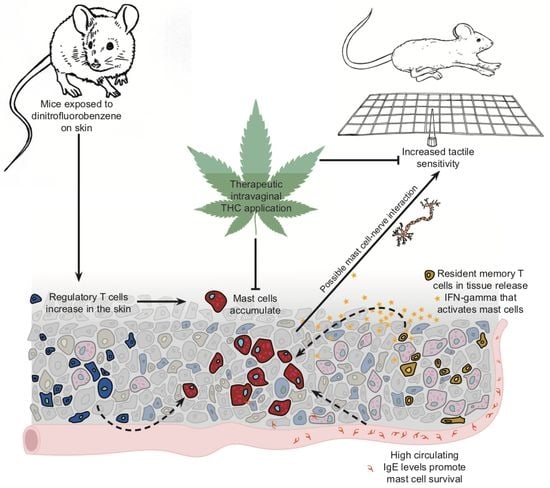
Tetrahydrocannabinol Reduces Hapten-Driven Mast Cell Accumulation and Persistent Tactile Sensitivity in Mouse Model of Allergen-Provoked Localized Vulvodynia
Abstract
:1. Introduction
2. Results
2.1. Ten Exposures to Dinitrofluorobenzene (DNFB) Dissolved in Saline on the Labiar Skin of Previously Sensitized ND4 Swiss Mice Induce Local and Systemic Inflammatory Responses
2.2. Ten DNFB Administrations on the Labiar Skin, or in the Vaginal Canal, of Previously Sensitized ND4 Swiss Mice Provoke Persistent Vulvar Mechanical Tactile Sensitivity
2.3. Ten DNFB Exposures into the Vaginal Canals of Previously Sensitized ND4 Swiss Mice Induce Local and Systemic Inflammatory Responses
2.4. Repeated Δ9-Tetrahydrocannabinol (THC) Application Reduces Tactile Sensitivity and Mast Cell Density after 10 Exposures to DNFB in the Vaginal Canals of Previously Sensitized ND4 Mice
3. Discussion
4. Materials and Methods
4.1. Animal Usage
4.2. DNFB Administration: Sensitization and Challenge
4.2.1. Labiar and Vaginal Canal Challenges
4.2.2. THC Treatment
4.2.3. Electronic Von Frey Measurements
4.2.4. Tissue Collection
4.2.5. RNA Extraction and Quantitative Real-Time Reverse Transcriptase PCR (qRT-PCR)
4.2.6. Immunofluorescent Staining of Mast Cells and Nerves
4.2.7. Confocal Imaging
4.2.8. Quantification of Local Eosinophil Peroxidase (EPO) and Myeloperoxidase (MPO) Activity
4.2.9. Lymph Node Processing and Flow Cytometry
4.2.10. Flank Skin Processing and Flow Cytometry
4.2.11. IgE Enzyme-Linked Immunosorbent Assay (ELISA)
4.2.12. Data Analysis and Visualization
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Pukall, C.F.; Goldstein, A.T.; Bergeron, S.; Foster, D.; Stein, A.; Kellogg-Spadt, S.; Bachmann, G. Vulvodynia: Definition, Prevalence, Impact, and Pathophysiological Factors. J. Sex Med. 2016, 13, 291–304. [Google Scholar] [CrossRef]
- Sadownik, L.A. Etiology, diagnosis, and clinical management of vulvodynia. Int. J. Womens Health 2014, 6, 437–449. [Google Scholar] [CrossRef]
- Bornstein, J.; Goldschmid, N.; Sabo, E. Hyperinnervation and mast cell activation may be used as histopathologic diagnostic criteria for vulvar vestibulitis. Gynecol. Obstet. Investig. 2004, 58, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Falsetta, M.L.; Foster, D.C.; Woeller, C.F.; Pollock, S.J.; Bonham, A.D.; Haidaris, C.G.; Stodgell, C.J.; Phipps, R.P. Identification of novel mechanisms involved in generating localized vulvodynia pain. Am. J. Obstet. Gynecol. 2015, 213, 38.e1-e12. [Google Scholar] [CrossRef] [PubMed]
- Foster, D.C.; Piekarz, K.H.; Murant, T.I.; LaPoint, R.; Haidaris, C.G.; Phipps, R.P. Enhanced synthesis of proinflammatory cytokines by vulvar vestibular fibroblasts: Implications for vulvar vestibulitis. Am. J. Obstet. Gynecol. 2007, 196, 346.e1-e8. [Google Scholar] [CrossRef] [PubMed]
- Harlow, B.L.; He, W.; Nguyen, R.H. Allergic reactions and risk of vulvodynia. Ann. Epidemiol. 2009, 19, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Reed, B.D.; McKee, K.S.; Plegue, M.A.; Park, S.K.; Haefner, H.K.; Harlow, S.D. Environmental Exposure History and Vulvodynia Risk: A Population-Based Study. J. Womens Health 2019, 28, 69–76. [Google Scholar] [CrossRef]
- Landry, J.; Martinov, T.; Mengistu, H.; Dhanwada, J.; Benck, C.J.; Kline, J.; Boo, B.; Swanson, L.; Tonc, E.; Daughters, R.; et al. Repeated hapten exposure induces persistent tactile sensitivity in mice modeling localized provoked vulvodynia. PLoS ONE 2017, 12, e0169672. [Google Scholar] [CrossRef] [PubMed]
- Martinov, T.; Glenn-Finer, R.; Burley, S.; Tonc, E.; Balsells, E.; Ashbaugh, A.; Swanson, L.; Daughters, R.S.; Chatterjea, D. Contact hypersensitivity to oxazolone provokes vulvar mechanical hyperalgesia in mice. PLoS ONE 2013, 8, e78673. [Google Scholar] [CrossRef]
- Dudeck, A.; Dudeck, J.; Scholten, J.; Petzold, A.; Surianarayanan, S.; Köhler, A.; Peschke, K.; Vöhringer, D.; Waskow, C.; Krieg, T.; et al. Mast cells are key promoters of contact allergy that mediate the adjuvant effects of haptens. Immunity 2011, 24, 973–984. [Google Scholar] [CrossRef]
- Kraneveld, A.D.; van der Kleij, H.P.; Kool, M.; van Houwelingen, A.H.; Weitenberg, A.C.; Redegeld, F.A.; Nijkamp, F.P. Key role for mast cells in nonatopic asthma. J. Immunol. 2002, 169, 2044–2053. [Google Scholar] [CrossRef]
- Dudeck, J.; Medyukhina, A.; Fröbel, J.; Svensson, C.M.; Kotrba, J.; Gerlach, M.; Gradtke, A.C.; Schröder, B.; Speier, S.; Figge, M.T.; et al. Mast cells acquire MHCII from dendritic cells during skin inflammation. J. Exp. Med. 2017, 4, 3791–3811. [Google Scholar] [CrossRef]
- Honda, T.; Egawa, G.; Grabbe, S.; Kabahima, K. Update of immune events in the murine contact hypersensitivity model: Towards the understanding of allergic contact dermatitis. J. Investig. Dermatol. 2013, 133, 303–315. [Google Scholar] [CrossRef]
- Schneider, C.; Döcke, W.D.; Zollner, T.M.; Röse, L. Chronic mouse model of TMA-induced contact hypersensitivity. J. Investig. Dermatol. 2009, 129, 899–907. [Google Scholar] [CrossRef]
- Yu, M.; Eckart, M.R.; Morgan, A.A.; Mukai, K.; Butte, A.J.; Tsai, M.; Galli, S.J. Identification of an IFN-γ/mast cell axis in a mouse model of chronic asthma. J. Clin. Investig. 2011, 121, 3133–3143. [Google Scholar] [CrossRef] [Green Version]
- Gaffal, E.; Cron, M.; Glodde, N.; Tüting, T. Anti-inflammatory activity of topical THC in DNFB-mediated mouse allergic contact dermatitis independent of CB1 and CB2 receptors. Allergy 2013, 68, 994–1000. [Google Scholar] [CrossRef]
- Nam, G.; Jeong, S.K.; Park, B.M.; Lee, S.H.; Kim, H.J.; Hong, S.P.; Kim, B.; Kim, B.W. Selective Cannabinoid Receptor-1 Agonists Regulate Mast Cell Activation in an Oxazolone-Induced Atopic Dermatitis Model. Ann. Dermatol. 2016, 28, 22–29. [Google Scholar] [CrossRef] [Green Version]
- Chatterjea, D.; Martinov, T. Mast cells: Versatile gatekeepers of pain. Mol. Immunol. 2015, 63, 38–44. [Google Scholar] [CrossRef] [Green Version]
- Galli, S.J.; Kalesnikoff, J.; Grimbaldeston, M.A.; Piliponsky, A.M.; Williams, C.M.; Tsai, M. Mast cells as “tunable” effector and immunoregulatory cells: Recent advances. Annu. Rev. Immunol. 2005, 23, 749–786. [Google Scholar] [CrossRef]
- Chatterjea, D.; Wetzel, A.; Mack, M.; Engblom, C.; Allen, J.; Mora-Solano, C.; Paredes, L.; Balsells, E.; Martinov, T. Mast cell degranulation mediates compound 48/80-induced hyperalgesia in mice. Biochem. Biophys. Res. Commun. 2012, 425, 237–243. [Google Scholar] [CrossRef] [Green Version]
- Mack, M.; Tonc, E.; Ashbaugh, A.; Wetzel, A.; Sykes, A.; Engblom, C.; Shabani, E.; Mora-Solano, C.; Trier, A.; Swanson, L.; et al. Clonal differences in IgE antibodies affect cutaneous anaphylaxis-associated thermal sensitivity in mice. Immunol. Lett. 2014, 162, 149–158. [Google Scholar] [CrossRef] [Green Version]
- Levy, D. Migraine pain, meningeal inflammation, and mast cells. Curr. Pain Headache Rep. 2009, 13, 237–240. [Google Scholar] [CrossRef]
- Vincent, L.; Vang, D.; Nguyen, J.; Gupta, M.; Luk, K.; Ericson, M.E.; Simone, D.A.; Gupta, K. Mast cell activation contributes to sickle cell pathobiology and pain in mice. Blood 2013, 122, 1853–1862. [Google Scholar] [CrossRef] [Green Version]
- Ng, Q.X.; Soh, A.Y.S.; Loke, W.; Lim, D.Y.; Yeo, W.S. The role of inflammation in irritable bowel syndrome (IBS). J. Inflamm. Res. 2018, 11, 345–349. [Google Scholar] [CrossRef]
- Kaur, G.; Singh, N.; Jaggi, A.S. Mast cells in neuropathic pain: An increasing spectrum of their involvement in pathophysiology. Rev. Neurosci. 2017, 28, 759–766. [Google Scholar] [CrossRef]
- Hendriksen, E.; van Bergeijk, D.; Oosting, R.S.; Redegeld, F.A. Mast cells in neuroinflammation and brain disorders. Neurosci. Biobehav. Rev. 2017, 79, 119–133. [Google Scholar] [CrossRef]
- Bornstein, J.; Cohen, Y.; Zarfati, D.; Sela, S.; Ophir, E. Involvement of heparanase in the pathogenesis of localized vulvodynia. Int. J. Gynecol. Pathol. 2008, 27, 136–141. [Google Scholar] [CrossRef]
- Papoutsis, D.; Haefner, H.K.; Crum, C.P.; Opipari, A.W., Jr.; Reed, B.D. Vestibular mast cell density in vulvodynia: A case-controlled study. J. Low. Genit. Tract. Dis. 2016, 20, 275–279. [Google Scholar] [CrossRef]
- Vieira-Baptista, P.; Lima-Silva, J.; Pérez-López, F.R.; Preti, M.; Bornstein, J. Vulvodynia: A disease commonly hidden in plain sight. Case Rep. Womens Health 2018, 20, e00079. [Google Scholar] [CrossRef]
- Harlow, B.L.; Caron, R.E.; Parker, S.E.; Chatterjea, D.; Fox, M.P.; Nguyen, R.H.N. Recurrent Yeast Infections and Vulvodynia: Can We Believe Associations Based on Self-Reported Data? J. Womens Health (Larchmt) 2017, 26, 1069–1076. [Google Scholar] [CrossRef]
- Nguyen, R.H.; Swanson, D.; Harlow, B.L. Urogenital infections in relation to the occurrence of vulvodynia. J. Reprod. Med. 2009, 54, 385–392. [Google Scholar]
- Khandker, M.; Brady, S.S.; Vitonis, A.F.; Maclehose, R.F.; Stewart, E.G.; Harlow, B.L. The influence of depression and anxiety on risk of adult onset vulvodynia. J. Womens Health (Larchmt) 2011, 20, 1445–1451. [Google Scholar] [CrossRef]
- Jones, T.G.; Finkelman, F.D.; Austen, K.F.; Gurish, M.F. T regulatory cells control antigen-induced recruitment of mast cell progenitors to the lungs of C57BL/6 mice. J. Immunol. 2010, 185, 1804–1811. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Tsai, M.; Tam, S.Y.; Jones, C.; Zehnder, J.; Galli, S.J. Mast cells can promote the development of multiple features of chronic asthma in mice. J. Clin. Investig. 2006, 116, 1633–1641. [Google Scholar] [CrossRef] [Green Version]
- Schenkel, J.M.; Fraser, K.A.; Vezys, V.; Masopust, D. Sensing and alarm function of resident memory CD8⁺ T cells. Nat. Immunol. 2013, 14, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Asai, K.; Kitaura, J.; Kawakami, Y.; Yamagata, N.; Tsai, M.; Carbone, D.P.; Liu, F.T.; Galli, S.J.; Kawakami, T. Regulation of mast cell survival by IgE. Immunity 2001, 14, 791–800. [Google Scholar] [CrossRef]
- Bax, H.J.; Keeble, A.H.; Gould, H.J. Cytokinergic IgE action in mast cell activation. Front. Immunol. 2012, 3, 229. [Google Scholar] [CrossRef]
- Wang, H.W.; Tedla, N.; Lloyd, A.R.; Wakefield, D.; McNeil, P.H. Mast cell activation and migration to lymph nodes during induction of an immune response in mice. J. Clin. Investig. 1998, 102, 1617–1626. [Google Scholar] [CrossRef]
- Forsythe, P.; Bienenstock, J. The mast cell-nerve functional unit: A key component of physiologic and pathophysiologic responses. Chem. Immunol. Allergy 2012, 98, 196–221. [Google Scholar]
- Skaper, S.D. Nerve Growth factor: A neuroimmune crosstalk mediator for all seasons. Immunology 2017, 151, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Hagiyama, M.; Inoue, T.; Furuno, T.; Iino, T.; Itami, S.; Nakanishi, M.; Asada, H.; Hosokawa, Y.; Ito, A. Increased expression of cell adhesion molecule 1 by mast cells as a cause of enhanced nerve-mast cell interaction in a hapten-induced mouse model of atopic dermatitis. Br. J. Dermatol. 2013, 168, 771–778. [Google Scholar] [CrossRef]
- Nyirjesy, P.; Sobel, J.; Weitz, M.; Leaman, D.; Small, M.; Gelone, S. Cromolyn cream for recalcitrant idiopathic vulvar vestibulitis: Results of a placebo controlled study. Sex Transm. Infect. 2001, 77, 53–57. [Google Scholar] [CrossRef]
- Starowicz, K.; Finn, D.P. Cannabinoids and Pain: Sites and Mechanisms of Action. Adv. Pharmacol. 2017, 80, 437–475. [Google Scholar]
- Vincent, L.; Vang, D.; Nguyen, J.; Benson, B.; Lei, J.; Gupta, K. Cannabinoid receptor-specific mechanisms to alleviate pain in sickle cell anemia via inhibition of mast cell activation and neurogenic inflammation. Haematologica 2016, 101, 566–577. [Google Scholar] [CrossRef]
- Singh, U.; Singh, N.; Singh, B.; Price, R.; Nagarkatti, M.; Nagarkatti, P. Cannabinoid receptor-2 (CB2) agonist ameliorates colitis in IL-10(−/−) mice by attenuating the activation of T cells and promoting their apoptosis. Toxicol. Appl. Pharmacaol. 2012, 258, 256–267. [Google Scholar] [CrossRef]
- Khan, S.P.; Pickens, T.A.; Berlau, D.J. Perspectives on cannabis as a substitute for opioid analgesics. Pain Manag. 2019, 9, 191–203. [Google Scholar] [CrossRef]
- Aviram, J.; Samuelly-Leichtag, G. Efficacy of cannabis-based medicines for pain management: A systematic review and meta-analysis of randomized controlled trials. Pain Physician 2017, 20, E755–E796. [Google Scholar] [PubMed]
- Martinov, T.; Mack, M.; Sykes, A.; Chatterjea, D. Measuring changes in tactile sensitivity in the hind paw of mice using an electronic von Frey apparatus. J. Vis. Exp. 2013, 19, e51212. [Google Scholar] [CrossRef] [PubMed]
- Farmer, M.A.; Taylor, A.M.; Bailey, A.L.; Tuttle, A.H.; MacIntyre, L.C.; Milagrosa, Z.E.; Crissman, H.P.; Bennett, G.J.; Ribeiro-da-Silva, A.; Binik, Y.M.; et al. Repeated vulvovaginal fungal infections cause persistent pain in a mouse model of vulvodynia. Sci. Transl. Med. 2011, 3, 101ra91. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Benck, C.J.; Martinov, T.; Fife, B.T.; Chatterjea, D. Isolation of Infiltrating Leukocytes from Mouse Skin Using Enzymatic Digest and Gradient Separation. J. Vis. Exp. 2016, 107, e53638. [Google Scholar] [CrossRef] [PubMed]





| Antigen | Color | Clone | Host | Stable Public Identifier | Vendor |
|---|---|---|---|---|---|
| CD3 | BV421 | 145-2C11 | Armenian Hamster | AB_2562556 | BioLegend |
| CD4 | PECy7 | RM4-5 | Rat | AB_2621829 | Tonbo Biosciences |
| CD8 | APC | 53-6.7 | Rat | AB_2621550 | Tonbo Biosciences |
| CD19 | BV785 | 6D5 | Rat | AB_11218994 | BioLegend |
| Ckit | PE | ACK2 | Rat | AB_2621506 | Tonbo Biosciences |
| FcεR1 | FITC | MAR-1 | Hamster | AB_10829796 | Miltenyi Biotec |
| Ghost Dye | BV510 | - | - | - | Tonbo Biosciences |
| Antigen | Color | Clone | Host | Stable Public Identifier | Vendor |
|---|---|---|---|---|---|
| CD45.1 | APC efluor 780 | A20 | Mouse | AB_1582228 | Thermo Fisher |
| CD45.2 | APC efluor 780 | 104 | Mouse | AB_1272175 | Thermo Fisher |
| CD3 | BUV395 | 145-2C11 | Armenian Hamster | - | BD Biosciences |
| CD19 | BV786 | 6D5 | Rat | AB_11218994 | BioLegend |
| CD25 | BV650 | PC61 | Rat | AB_11125760 | BioLegend |
| CD8 | APC | 53-6.7 | Rat | AB_2621550 | Tonbo Biosciences |
| CD4 | PerCP Cy5.5 | RM4-5 | Rat | AB_2621876 | Tonbo Biosciences |
| CD44 | efluor 450 | IM7 | Rat | AB_1272250 | Thermo Fisher |
| CD103 | FITC | 2E7 | Armenian Hamster | AB_10709438 | BioLegend |
| Ghost Dye | BV510 | - | - | - | Tonbo Biosciences |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boo, B.; Kamath, R.; Arriaga-Gomez, E.; Landry, J.; Emanuel, E.; Joo, S.; Saldías Montivero, M.; Martinov, T.; Fife, B.T.; Chatterjea, D. Tetrahydrocannabinol Reduces Hapten-Driven Mast Cell Accumulation and Persistent Tactile Sensitivity in Mouse Model of Allergen-Provoked Localized Vulvodynia. Int. J. Mol. Sci. 2019, 20, 2163. https://doi.org/10.3390/ijms20092163
Boo B, Kamath R, Arriaga-Gomez E, Landry J, Emanuel E, Joo S, Saldías Montivero M, Martinov T, Fife BT, Chatterjea D. Tetrahydrocannabinol Reduces Hapten-Driven Mast Cell Accumulation and Persistent Tactile Sensitivity in Mouse Model of Allergen-Provoked Localized Vulvodynia. International Journal of Molecular Sciences. 2019; 20(9):2163. https://doi.org/10.3390/ijms20092163
Chicago/Turabian StyleBoo, Beebie, Rohit Kamath, Erica Arriaga-Gomez, Jasmine Landry, Elizabeth Emanuel, Sookyong Joo, Marietta Saldías Montivero, Tijana Martinov, Brian T. Fife, and Devavani Chatterjea. 2019. "Tetrahydrocannabinol Reduces Hapten-Driven Mast Cell Accumulation and Persistent Tactile Sensitivity in Mouse Model of Allergen-Provoked Localized Vulvodynia" International Journal of Molecular Sciences 20, no. 9: 2163. https://doi.org/10.3390/ijms20092163
APA StyleBoo, B., Kamath, R., Arriaga-Gomez, E., Landry, J., Emanuel, E., Joo, S., Saldías Montivero, M., Martinov, T., Fife, B. T., & Chatterjea, D. (2019). Tetrahydrocannabinol Reduces Hapten-Driven Mast Cell Accumulation and Persistent Tactile Sensitivity in Mouse Model of Allergen-Provoked Localized Vulvodynia. International Journal of Molecular Sciences, 20(9), 2163. https://doi.org/10.3390/ijms20092163