Glomerular Hematuria: Cause or Consequence of Renal Inflammation?
Abstract
:1. Introduction
2. Glomerular Hematuria: An Important and Often-Neglected Clinical Sign
2.1. Prevalence of Glomerular Hematuria
2.2. Common Causes of Glomerular Hematuria
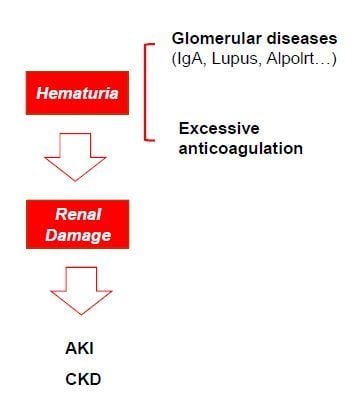
3. Hematuria and Renal Damage: Cause or Consequence?
3.1. Hematuria as a Sign of Glomerular Inflammation and Disease Progression
3.2. Pathophysiological Consequences of Hematuria (AKI and CKD)
3.2.1. Hematuria and AKI
3.2.2. Hematuria as a Risk Factor for CKD
4. Should Hematuria be Included as a Surrogate Marker in Clinical Trials of Renal Diseases?
5. Hematuria at the Crossroads of Inflammation and Oxidative Stress
6. Can We Envision a Therapeutic Approach for Hematuria-Associated Diseases?
6.1. Treatment for the Prevention of Gross Hematuria Bouts
6.2. Treatment of the Nephrotoxic Effect of Hematuria
7. Conclusions
Funding
Conflicts of Interest
References
- Woolhandler, S.; Pels, R.J.; Bor, D.H.; Himmelstein, D.U.; Lawrence, R.S. Dipstick urinalysis screening of asymptomatic adults for urinary tract disorders. I. Hematuria and proteinuria. JAMA 1989, 262, 1214–1219. [Google Scholar] [CrossRef] [PubMed]
- Hiatt, R.A.; Ordoñez, J.D. Dipstick urinalysis screening, asymptomatic microhematuria, and subsequent urological cancers in a population-based sample. Cancer Epidemiol. Biomarkers Prev. 1994, 3, 439–443. [Google Scholar]
- Ramirez, S.P.B.; Kapke, A.; Port, F.K.; Wolfe, R.A.; Saran, R.; Pearson, J.; Hirth, R.A.; Messana, J.M.; Daugirdas, J.T. Dialysis Dose Scaled to Body Surface Area and Size-Adjusted, Sex-Specific Patient Mortality. Clin. J. Am. Soc. Nephrol. 2012, 7, 1977–1987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chadban, S.J.; Briganti, E.M.; Kerr, P.G.; Dunstan, D.W.; Welborn, T.A.; Zimmet, P.Z.; Atkins, R.C. Prevalence of kidney damage in Australian adults: The AusDiab kidney study. J. Am. Soc. Nephrol. 2003, 14, S131–S138. [Google Scholar] [CrossRef] [PubMed]
- Ong, L.M.; Punithavathi, N.; Thurairatnam, D.; Zainal, H.; Beh, M.L.; Morad, Z.; Lee, S.Y.; Bavanandan, S.; Kok, L.S. Prevalence and risk factors for proteinuria: The National Kidney Foundation of Malaysia Lifecheck Health Screening programme. Nephrology 2013, 18, 569–575. [Google Scholar] [CrossRef]
- Vivante, A.; Afek, A.; Frenkel-Nir, Y.; Tzur, D.; Farfel, A.; Golan, E.; Chaiter, Y.; Shohat, T.; Skorecki, K.; Calderon-Margalit, R. Persistent asymptomatic isolated microscopic hematuria in Israeli adolescents and young adults and risk for end-stage renal disease. JAMA 2011, 306, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Hajar, F.; Taleb, M.; Aoun, B.; Shatila, A. Dipstick urine analysis screening among asymptomatic school children. N. Am. J. Med. Sci. 2011, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Liu, Q.; Wang, H.; Chen, W.; Johnson, R.J.; Dong, X.; Li, H.; Ba, S.; Tan, J.; Luo, N.; et al. Prevalence and risk factors of chronic kidney disease: A population study in the Tibetan population. Nephrol. Dial. Trans. 2011, 26, 1592–1599. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Hayakawa, M.; Yanagihara, T.; Hukunaga, Y. Proteinuria screening for children. Kidney Int. Suppl. 2005, S23–S27. [Google Scholar] [CrossRef]
- Cohen, R.A.; Brown, R.S. Clinical practice. Microscopic hematuria. N. Engl. J. Med. 2003, 348, 2330–2338. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.A.; Martin-Cleary, C.; Gutierrez, E.; Rubio-Navarro, A.; Ortiz, A.; Praga, M.; Egido, J. Haematuria: The forgotten CKD factor? Nephrol. Dial. Trans. 2012, 27, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Rychlik, I.; Jancova, E.; Tesar, V.; Kolsky, A.; Lacha, J.; Stejskal, J.; Stejskalova, A.; Dusek, J.; Herout, V. The Czech registry of renal biopsies. Occurrence of renal diseases in the years 1994-2000. Nephrol. Dial. Trans. 2004, 19, 3040–3049. [Google Scholar] [CrossRef] [Green Version]
- Maixnerova, D.; Jancova, E.; Skibova, J.; Rysava, R.; Rychlik, I.; Viklicky, O.; Merta, M.; Kolsky, A.; Reiterova, J.; Neprasova, M.; et al. Nationwide biopsy survey of renal diseases in the Czech Republic during the years 1994–2011. J. Nephrol. 2015, 28, 39–49. [Google Scholar] [CrossRef]
- Schena, F.P. Survey of the Italian Registry of Renal Biopsies. Frequency of the renal diseases for 7 consecutive years. The Italian Group of Renal Immunopathology. Nephrol. Dial. Trans. 1997, 12, 418–426. [Google Scholar] [CrossRef] [Green Version]
- Paripović, D.; Kostić, M.; Kruščić, D.; Spasojević, B.; Lomić, G.; Marković-Lipkovski, J.; Basta-Jovanović, G.; Smoljanić, Ž.; Peco-Antić, A. Indications and results of renal biopsy in children: A 10-year review from a single center in Serbia. J. Nephrol. 2012, 25, 1054–1059. [Google Scholar] [CrossRef] [PubMed]
- Polito, M.G.; de Moura, L.A.R.; Kirsztajn, G.M. An overview on frequency of renal biopsy diagnosis in Brazil: Clinical and pathological patterns based on 9617 native kidney biopsies. Nephrol. Dial. Trans. 2010, 25, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Yuste, C.; Rivera, F.; Moreno, J.A.; López-Gómez, J.M. Haematuria on the Spanish Registry of Glomerulonephritis. Sci. Rep. 2016, 6, 19732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haas, M.; Racusen, L.C.; Bagnasco, S.M. IgA-dominant postinfectious glomerulonephritis: A report of 13 cases with common ultrastructural features. Hum. Pathol. 2008, 39, 1309–1316. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.Y.C.; Ibañez, D.; Gladman, D.D.; Urowitz, M.B. Isolated Hematuria and Sterile Pyuria May Indicate Systemic Lupus Erythematosus Activity. J. Rheumatol. 2015, 42, 437–440. [Google Scholar] [CrossRef] [PubMed]
- Rhee, R.L.; Davis, J.C.; Ding, L.; Fervenza, F.C.; Hoffman, G.S.; Kallenberg, C.G.M.; Langford, C.A.; McCune, W.J.; Monach, P.A.; Seo, P.; et al. The Utility of Urinalysis in Determining the Risk of Renal Relapse in ANCA-Associated Vasculitis. Clin. J. Am. Soc. Nephrol. 2018, 13, 251–257. [Google Scholar] [CrossRef]
- Gutiérrez, E.; González, E.; Hernández, E.; Morales, E.; Martínez, M.A.; Usera, G.; Praga, M. Factors that determine an incomplete recovery of renal function in macrohematuria-induced acute renal failure of IgA nephropathy. Clin. J. Am. Soc. Nephrol. 2007, 2, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Sevillano, A.M.; Gutiérrez, E.; Yuste, C.; Cavero, T.; Mérida, E.; Rodríguez, P.; García, A.; Morales, E.; Fernández, C.; Martínez, M.A.; et al. Remission of Hematuria Improves Renal Survival in IgA Nephropathy. J. Am. Soc. Nephrol. 2017, 28, 3089–3099. [Google Scholar] [CrossRef]
- Moroni, G.; Pozzi, C.; Quaglini, S.; Segagni, S.; Banfi, G.; Baroli, A.; Picardi, L.; Colzani, S.; Simonini, P.; Mihatsch, M.J.; et al. Long-term prognosis of diffuse proliferative glomerulonephritis associated with infection in adults. Nephrol. Dial. Trans. 2002, 17, 1204–1211. [Google Scholar] [CrossRef] [Green Version]
- Sethi, S.; Fervenza, F.C. Membranoproliferative Glomerulonephritis—A New Look at an Old Entity. N. Engl. J. Med. 2012, 366, 1119–1131. [Google Scholar] [CrossRef]
- Wyatt, R.J.; Julian, B.A. IgA Nephropathy. N. Engl. J. Med. 2013, 368, 2402–2414. [Google Scholar] [CrossRef]
- D’Agati, V.D.; Kaskel, F.J.; Falk, R.J. Focal Segmental Glomerulosclerosis. N. Engl. J. Med. 2011, 365, 2398–2411. [Google Scholar] [CrossRef]
- Sethi, S.; Zand, L.; Nasr, S.H.; Glassock, R.J.; Fervenza, F.C. Focal and segmental glomerulosclerosis: Clinical and kidney biopsy correlations. Clin. Kidney J. 2014, 7, 531–537. [Google Scholar] [CrossRef]
- Medjeral-Thomas, N.R.; O’Shaughnessy, M.M.; O’Regan, J.A.; Traynor, C.; Flanagan, M.; Wong, L.; Teoh, C.W.; Awan, A.; Waldron, M.; Cairns, T.; et al. C3 glomerulopathy: Clinicopathologic features and predictors of outcome. Clin. J. Am. Soc. Nephrol. 2014, 9, 46–53. [Google Scholar] [CrossRef]
- Pickering, M.C.; D’Agati, V.D.; Nester, C.M.; Smith, R.J.; Haas, M.; Appel, G.B.; Alpers, C.E.; Bajema, I.M.; Bedrosian, C.; Braun, M.; et al. C3 glomerulopathy: Consensus report. Kidney Int. 2013, 84, 1079–1089. [Google Scholar] [CrossRef]
- Kovačević, Z.; Jovanović, D.; Rabrenović, V.; Dimitrijević, J.; Djukanović, J. Asymptomatic microscopic haematuria in young males. Int. J. Clin. Pract. 2008, 62, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Deltas, C.; Pierides, A.; Voskarides, K. The role of molecular genetics in diagnosing familial hematuria(s). Pediatr. Nephrol. 2012, 27, 1221–1231. [Google Scholar] [CrossRef]
- Deltas, C.; Pierides, A.; Voskarides, K. Molecular genetics of familial hematuric diseases. Nephrol. Dial. Trans. 2013, 28, 2946–2960. [Google Scholar] [CrossRef] [Green Version]
- Kashtan, C.E. Familial hematuria. Pediatr. Nephrol. 2009, 24, 1951–1958. [Google Scholar] [CrossRef] [PubMed]
- Brodsky, S.V.; Satoskar, A.; Chen, J.; Nadasdy, G.; Eagen, J.W.; Hamirani, M.; Hebert, L.; Calomeni, E.; Nadasdy, T. Acute Kidney Injury During Warfarin Therapy Associated With Obstructive Tubular Red Blood Cell Casts: A Report of 9 Cases. Am. J. Kidney Dis. 2009, 54, 1121–1126. [Google Scholar] [CrossRef] [PubMed]
- Escoli, R.; Santos, P.; Andrade, S.; Carvalho, F. Dabigatran-Related Nephropathy in a Patient with Undiagnosed IgA Nephropathy. Case Rep. Nephrol. 2015, 2015, 298261. [Google Scholar] [CrossRef]
- Brodsky, S.V.; Mhaskar, N.S.; Thiruveedi, S.; Dhingra, R.; Reuben, S.C.; Calomeni, E.; Ivanov, I.; Satoskar, A.; Hemminger, J.; Nadasdy, G.; et al. Acute kidney injury aggravated by treatment initiation with apixaban: Another twist of anticoagulant-related nephropathy. Kidney Res. Clin. Pract. 2017, 36, 387–392. [Google Scholar] [CrossRef] [Green Version]
- Brodsky, S.; Eikelboom, J.; Hebert, L.A. Anticoagulant-Related Nephropathy. J. Am. Soc. Nephrol. 2018, 29, 2787–2793. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.-I.; Luo, S.; Alexander, G.C.; Inker, L.A.; Coresh, J.; Chang, A.R.; Grams, M.E. Direct Oral Anticoagulants and Risk of Acute Kidney Injury in Patients With Atrial Fibrillation. J. Am. Coll. Cardiol. 2018, 71, 251–252. [Google Scholar] [CrossRef]
- Chan, Y.-H.; Yeh, Y.-H.; See, L.-C.; Wang, C.-L.; Chang, S.-H.; Lee, H.-F.; Wu, L.-S.; Tu, H.-T.; Kuo, C.-T. Acute Kidney Injury in Asians With Atrial Fibrillation Treated With Dabigatran or Warfarin. J. Am. Coll. Cardiol. 2016, 68, 2272–2283. [Google Scholar] [CrossRef] [Green Version]
- Yao, X.; Tangri, N.; Gersh, B.J.; Sangaralingham, L.R.; Shah, N.D.; Nath, K.A.; Noseworthy, P.A. Renal Outcomes in Anticoagulated Patients With Atrial Fibrillation. J. Am. Coll. Cardiol. 2017, 70, 2621–2632. [Google Scholar] [CrossRef] [PubMed]
- Brodsky, S.V.; Nadasdy, T.; Rovin, B.H.; Satoskar, A.A.; Nadasdy, G.M.; Wu, H.M.; Bhatt, U.Y.; Hebert, L.A. Warfarin-related nephropathy occurs in patients with and without chronic kidney disease and is associated with an increased mortality rate. Kidney Int. 2011, 80, 181–189. [Google Scholar] [CrossRef] [Green Version]
- Lechner, S.M.; Abbad, L.; Boedec, E.; Papista, C.; Le Stang, M.-B.; Moal, C.; Maillard, J.; Jamin, A.; Bex-Coudrat, J.; Wang, Y.; et al. IgA1 Protease Treatment Reverses Mesangial Deposits and Hematuria in a Model of IgA Nephropathy. J. Am. Soc. Nephrol. 2016, 27, 2622–2629. [Google Scholar] [CrossRef] [Green Version]
- Robert, T.; Berthelot, L.; Cambier, A.; Rondeau, E.; Monteiro, R.C. Molecular Insights into the Pathogenesis of IgA Nephropathy. Trends Mol. Med. 2015, 21, 762–775. [Google Scholar] [CrossRef]
- Suzuki, Y.; Suzuki, H.; Nakata, J.; Sato, D.; Kajiyama, T.; Watanabe, T.; Tomino, Y. Pathological role of tonsillar B cells in IgA nephropathy. Clin. Dev. Immunol. 2011, 2011, 639074. [Google Scholar] [CrossRef]
- Meng, T.; Li, X.; Ao, X.; Zhong, Y.; Tang, R.; Peng, W.; Yang, J.; Zou, M.; Zhou, Q. Hemolytic Streptococcus may exacerbate kidney damage in IgA nephropathy through CCL20 response to the effect of Th17 cells. PLoS ONE 2014, 9, e108723. [Google Scholar] [CrossRef]
- Nishikawa, Y.; Shibata, R.; Ozono, Y.; Ichinose, H.; Miyazaki, M.; Harada, T.; Kohno, S. Streptococcal M protein enhances TGF-beta production and increases surface IgA-positive B cells in vitro in IgA nephropathy. Nephrol. Dial. Trans. 2000, 15, 772–777. [Google Scholar] [CrossRef]
- Barratt, J.; Smith, A.C.; Feehally, J. The pathogenic role of IgA1 O-linked glycosylation in the pathogenesis of IgA nephropathy. Nephrology (Carlton) 2007, 12, 275–284. [Google Scholar] [CrossRef]
- Cox, S.N.; Sallustio, F.; Serino, G.; Loverre, A.; Pesce, F.; Gigante, M.; Zaza, G.; Stifanelli, P.F.; Ancona, N.; Schena, F.P. Activated innate immunity and the involvement of CX3CR1-fractalkine in promoting hematuria in patients with IgA nephropathy. Kidney Int. 2012, 82, 548–560. [Google Scholar] [CrossRef]
- Nakatani, K.; Yoshimoto, S.; Iwano, M.; Asai, O.; Samejima, K.; Sakan, H.; Terada, M.; Hasegawa, H.; Nose, M.; Saito, Y. Fractalkine expression and CD16+ monocyte accumulation in glomerular lesions: Association with their severity and diversity in lupus models. Am. J. Physiol. Renal Physiol. 2010, 299, F207–F216. [Google Scholar] [CrossRef]
- Ayoub, I.; Birmingham, D.; Rovin, B.; Hebert, L. Commentary on the Current Guidelines for the Diagnosis of Lupus Nephritis Flare. Curr. Rheumatol. Rep. 2019, 21, 12. [Google Scholar] [CrossRef]
- Moreno, J.A.; Martin-Cleary, C.; Gutierrez, E.; Toldos, O.; Blanco-Colio, L.M.; Praga, M.; Ortiz, A.; Egido, J. AKI Associated with Macroscopic Glomerular Hematuria: Clinical and Pathophysiologic Consequences. Clin. J. Am. Soc. Nephrol. 2012, 7, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, E.; Egido, J.; Rubio-Navarro, A.; Buendía, I.; Blanco Colio, L.M.; Toldos, O.; Manzarbeitia, F.; de Lorenzo, A.; Sanchez, R.; Ortiz, A.; et al. Oxidative stress, macrophage infiltration and CD163 expression are determinants of long-term renal outcome in macrohematuria-induced acute kidney injury of IgA nephropathy. Nephron. Clin. Pract. 2012, 121, c42–53. [Google Scholar] [CrossRef]
- Rubio-Navarro, A.; Sanchez-Niño, M.D.; Guerrero-Hue, M.; García-Caballero, C.; Gutiérrez, E.; Yuste, C.; Sevillano, Á.; Praga, M.; Egea, J.; Román, E.; et al. Podocytes are new cellular targets of haemoglobin-mediated renal damage. J. Pathol. 2018, 244. [Google Scholar] [CrossRef]
- Duan, Z.-Y.; Cai, G.-Y.; Bu, R.; Lu, Y.; Hou, K.; Chen, X.-M. Selection of urinary sediment miRNAs as specific biomarkers of IgA nephropathy. Sci. Rep. 2016, 6, 23498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, Z.-Y.; Cai, G.-Y.; Li, J.-J.; Bu, R.; Chen, X.-M. Urinary Erythrocyte-Derived miRNAs: Emerging Role in IgA Nephropathy. Kidney Blood Press. Res. 2017, 42, 738–748. [Google Scholar] [CrossRef] [Green Version]
- Tan, K.; Chen, J.; Li, W.; Chen, Y.; Sui, W.; Zhang, Y.; Dai, Y. Genome-wide analysis of microRNAs expression profiling in patients with primary IgA nephropathy. Genome 2013, 56, 161–169. [Google Scholar] [CrossRef]
- Goto, M.; Wakai, K.; Kawamura, T.; Ando, M.; Endoh, M.; Tomino, Y. A scoring system to predict renal outcome in IgA nephropathy: A nationwide 10-year prospective cohort study. Nephrol. Dial. Trans. 2009, 24, 3068–3074. [Google Scholar] [CrossRef]
- Barraca, D.; Moreno, J.A.; Aragoncillo, I.; Praga, M.; Gutiérrez, E.; Vega, A.; Egido, J.; Rubio-Navarro, A.; Mahillo, I.; Santos, A.; et al. Haematuria Increases Progression of Advanced Proteinuric Kidney Disease. PLoS ONE 2015, 10, e0128575. [Google Scholar]
- Orlandi, P.F.; Fujii, N.; Roy, J.; Chen, H.Y.; Lee Hamm, L.; Sondheimer, J.H.; He, J.; Fischer, M.J.; Rincon-Choles, H.; Krishnan, G.; et al. Hematuria as a risk factor for progression of chronic kidney disease and death: Findings from the Chronic Renal Insufficiency Cohort (CRIC) Study. BMC Nephrol. 2018, 19, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Schulman, G.; Berl, T.; Beck, G.J.; Remuzzi, G.; Ritz, E.; Shimizu, M.; Kikuchi, M.; Shobu, Y. Risk factors for progression of chronic kidney disease in the EPPIC trials and the effect of AST-120. Clin. Exp. Nephrol. 2018, 22, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.Y.H.; Niu, S.W.; Kuo, I.C.; Lim, L.M.; Hwang, D.Y.; Lee, J.J.; Hwang, S.J.; Chen, H.C.; Hung, C.C. Hematuria and Renal Outcomes in Patients With Diabetic Chronic Kidney Disease. Am. J. Med. Sci. 2018, 356, 268–276. [Google Scholar] [CrossRef]
- Levey, A.S.; Cattran, D.; Friedman, A.; Miller, W.G.; Sedor, J.; Tuttle, K.; Kasiske, B.; Hostetter, T. Proteinuria as a surrogate outcome in CKD: report of a scientific workshop sponsored by the National Kidney Foundation and the US Food and Drug Administration. Am. J. Kidney Dis. 2009, 54, 205–226. [Google Scholar] [CrossRef] [PubMed]
- Berthoux, F.; Mohey, H.; Laurent, B.; Mariat, C.; Afiani, A.; Thibaudin, L. Predicting the Risk for Dialysis or Death in IgA Nephropathy. J. Am. Soc. Nephrol. 2011, 22, 752–761. [Google Scholar] [CrossRef] [Green Version]
- Le, W.; Liang, S.; Hu, Y.; Deng, K.; Bao, H.; Zeng, C.; Liu, Z. Long-term renal survival and related risk factors in patients with IgA nephropathy: Results from a cohort of 1155 cases in a Chinese adult population. Nephrol. Dial. Trans. 2012, 27, 1479–1485. [Google Scholar] [CrossRef] [PubMed]
- Coppo, R.; Fervenza, F.C. Persistent Microscopic Hematuria as a Risk Factor for Progression of IgA Nephropathy: New Floodlight on a Nearly Forgotten Biomarker. J. Am. Soc. Nephrol. 2017, 28, 2831–2834. [Google Scholar] [CrossRef] [PubMed]
- Tracz, M.J.; Alam, J.; Nath, K.A. Physiology and Pathophysiology of Heme: Implications for Kidney Disease. J. Am. Soc. Nephrol. 2007, 18, 414–420. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Bandyopadhyay, U. Free heme toxicity and its detoxification systems in human. Toxicol. Lett. 2005, 157, 175–188. [Google Scholar] [CrossRef]
- Vincent, S.H. Oxidative effects of heme and porphyrins on proteins and lipids. Semin. Hematol. 1989, 26, 105–113. [Google Scholar] [PubMed]
- TAPPEL, A.L. The mechanism of the oxidation of unsaturated fatty acids catalyzed by hematin compounds. Arch. Biochem. Biophys. 1953, 44, 378–395. [Google Scholar] [CrossRef]
- Aft, R.L.; Mueller, G.C. Hemin-mediated DNA strand scission. J. Biol. Chem. 1983, 258, 12069–12072. [Google Scholar]
- Dutra, F.F.; Bozza, M.T. Heme on innate immunity and inflammation. Front. Pharmacol. 2014, 5, 115. [Google Scholar] [CrossRef]
- Balla, G.; Jacob, H.S.; Eaton, J.W.; Belcher, J.D.; Vercellotti, G.M. Hemin: A possible physiological mediator of low density lipoprotein oxidation and endothelial injury. Arterioscler. Thromb. J. Vasc. Biol. 1991, 11, 1700–1711. [Google Scholar] [CrossRef]
- Wagener, F.A.; Feldman, E.; de Witte, T.; Abraham, N.G. Heme induces the expression of adhesion molecules ICAM-1, VCAM-1, and E selectin in vascular endothelial cells. Proc. Soc. Exp. Biol. Med. 1997, 216, 456–463. [Google Scholar] [CrossRef]
- Immenschuh, S.; Vijayan, V.; Janciauskiene, S.; Gueler, F. Heme as a Target for Therapeutic Interventions. Front. Pharmacol. 2017, 8, 146. [Google Scholar] [CrossRef]
- Belcher, J.D.; Chen, C.; Nguyen, J.; Milbauer, L.; Abdulla, F.; Alayash, A.I.; Smith, A.; Nath, K.A.; Hebbel, R.P.; Vercellotti, G.M. Heme triggers TLR4 signaling leading to endothelial cell activation and vaso-occlusion in murine sickle cell disease. Blood 2014, 123, 377–390. [Google Scholar] [CrossRef]
- Graça-Souza, A.V.; Arruda, M.A.B.; de Freitas, M.S.; Barja-Fidalgo, C.; Oliveira, P.L. Neutrophil activation by heme: Implications for inflammatory processes. Blood 2002, 99, 4160–4165. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, A.P.T.; Pinheiro, C.S.; Luna-Gomes, T.; Alves, L.R.; Maya-Monteiro, C.M.; Porto, B.N.; Barja-Fidalgo, C.; Benjamim, C.F.; Peters-Golden, M.; Bandeira-Melo, C.; et al. Leukotriene B4 mediates neutrophil migration induced by heme. J. Immunol. 2011, 186, 6562–6567. [Google Scholar] [CrossRef]
- Arruda, M.A.; Rossi, A.G.; de Freitas, M.S.; Barja-Fidalgo, C.; Graça-Souza, A. V Heme inhibits human neutrophil apoptosis: Involvement of phosphoinositide 3-kinase, MAPK, and NF-kappaB. J. Immunol. 2004, 173, 2023–2030. [Google Scholar] [CrossRef]
- Figueiredo, R.T.; Fernandez, P.L.; Mourao-Sa, D.S.; Porto, B.N.; Dutra, F.F.; Alves, L.S.; Oliveira, M.F.; Oliveira, P.L.; Graça-Souza, A.V.; Bozza, M.T. Characterization of heme as activator of Toll-like receptor 4. J. Biol. Chem. 2007, 282, 20221–20229. [Google Scholar] [CrossRef]
- Lin, T.; Sammy, F.; Yang, H.; Thundivalappil, S.; Hellman, J.; Tracey, K.J.; Warren, H.S. Identification of hemopexin as an anti-inflammatory factor that inhibits synergy of hemoglobin with HMGB1 in sterile and infectious inflammation. J. Immunol. 2012, 189, 2017–2022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef]
- Nath, K.A.; Belcher, J.D.; Nath, M.C.; Grande, J.P.; Croatt, A.J.; Ackerman, A.W.; Katusic, Z.S.; Vercellotti, G.M. Role of TLR4 signaling in the nephrotoxicity of heme and heme proteins. Am. J. Physiol. Renal Physiol. 2018, 314, F906–F914. [Google Scholar] [CrossRef]
- Komada, T.; Usui, F.; Kawashima, A.; Kimura, H.; Karasawa, T.; Inoue, Y.; Kobayashi, M.; Mizushina, Y.; Kasahara, T.; Taniguchi, S.; et al. Role of NLRP3 Inflammasomes for Rhabdomyolysis-induced Acute Kidney Injury. Sci. Rep. 2015, 5, 10901. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.M.; Hardy, C.M. Cocoa feeding and human lactose intolerance. Am. J. Clin. Nutr. 1989, 49, 840–844. [Google Scholar] [CrossRef] [Green Version]
- Fellström, B.C.; Barratt, J.; Cook, H.; Coppo, R.; Feehally, J.; de Fijter, J.W.; Floege, J.; Hetzel, G.; Jardine, A.G.; Locatelli, F.; et al. Targeted-release budesonide versus placebo in patients with IgA nephropathy (NEFIGAN): A double-blind, randomised, placebo-controlled phase 2b trial. Lancet 2017, 389, 2117–2127. [Google Scholar] [CrossRef]
- Floege, J.; Barbour, S.J.; Cattran, D.C.; Hogan, J.J.; Nachman, P.H.; Tang, S.C.W.; Wetzels, J.F.M.; Cheung, M.; Wheeler, D.C.; Winkelmayer, W.C.; et al. Management and treatment of glomerular diseases (part 1): Conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2019, 95, 268–280. [Google Scholar] [CrossRef]
- Ware, K.; Qamri, Z.; Ozcan, A.; Satoskar, A.A.; Nadasdy, G.; Rovin, B.H.; Hebert, L.A.; Nadasdy, T.; Brodsky, S. V N-acetylcysteine ameliorates acute kidney injury but not glomerular hemorrhage in an animal model of warfarin-related nephropathy. Am. J. Physiol. Renal Physiol. 2013, 304, F1421–F1427. [Google Scholar] [CrossRef] [PubMed]

| Disease | Significance |
|---|---|
| Lupus nephritis | Classical symptom Marker of activity [19] |
| ANCA-associated vasculitis | Classical symptom Marker of activity Marker of risk to relapse after response to therapy [20] |
| Disorders of collagen IV α345 | Classical symptom |
| IgAN | Classical symptom Marker of activity Probable implicated in progression of the disease Implicated in AKI associated to gross hematuria [21] Probable risk factor to progression to ESRD [22] |
| Other primary glomerulopathies | Classical symptom Marker of activity [19] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno, J.A.; Sevillano, Á.; Gutiérrez, E.; Guerrero-Hue, M.; Vázquez-Carballo, C.; Yuste, C.; Herencia, C.; García-Caballero, C.; Praga, M.; Egido, J. Glomerular Hematuria: Cause or Consequence of Renal Inflammation? Int. J. Mol. Sci. 2019, 20, 2205. https://doi.org/10.3390/ijms20092205
Moreno JA, Sevillano Á, Gutiérrez E, Guerrero-Hue M, Vázquez-Carballo C, Yuste C, Herencia C, García-Caballero C, Praga M, Egido J. Glomerular Hematuria: Cause or Consequence of Renal Inflammation? International Journal of Molecular Sciences. 2019; 20(9):2205. https://doi.org/10.3390/ijms20092205
Chicago/Turabian StyleMoreno, Juan Antonio, Ángel Sevillano, Eduardo Gutiérrez, Melania Guerrero-Hue, Cristina Vázquez-Carballo, Claudia Yuste, Carmen Herencia, Cristina García-Caballero, Manuel Praga, and Jesús Egido. 2019. "Glomerular Hematuria: Cause or Consequence of Renal Inflammation?" International Journal of Molecular Sciences 20, no. 9: 2205. https://doi.org/10.3390/ijms20092205
APA StyleMoreno, J. A., Sevillano, Á., Gutiérrez, E., Guerrero-Hue, M., Vázquez-Carballo, C., Yuste, C., Herencia, C., García-Caballero, C., Praga, M., & Egido, J. (2019). Glomerular Hematuria: Cause or Consequence of Renal Inflammation? International Journal of Molecular Sciences, 20(9), 2205. https://doi.org/10.3390/ijms20092205