Consumption of Nitrate-Rich Beetroot Juice with or without Vitamin C Supplementation Increases the Excretion of Urinary Nitrate, Nitrite, and N-nitroso Compounds in Humans
Abstract
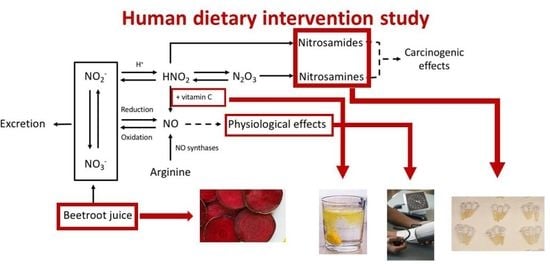
:1. Introduction
2. Results
2.1. Baseline Characteristics
2.2. ATNC Levels in Urine
2.3. Nitrate Levels in Urine
2.4. Nitrite Levels in Urine
2.5. Vitamin C Levels in Urine
2.6. Blood Pressure
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. Study Design
4.3. Analyses of Nitrate and Nitrite in Urine
4.4. Analysis of Vitamin C in Urine
4.5. Analyses of Creatinine Levels in Urine
4.6. Analysis of Apparent Total Nitroso Compounds (ATNC) in Urine
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BRJ | beetroot juice |
| NO | nitric oxide |
| BP | blood pressure |
| NOC | N-nitroso compound |
| ADI | acceptable daily intake |
| ATNC | apparent total N-nitroso compounds |
| CLD | chemiluminescence detector |
| NDMA | N-nitrosodimethylamine |
References
- Habermeyer, M.; Roth, A.; Guth, S.; Diel, P.; Engel, K.H.; Epe, B.; Furst, P.; Heinz, V.; Humpf, H.U.; Joost, H.G.; et al. Nitrate and nitrite in the diet: how to assess their benefit and risk for human health. Mol. Nutr. Food Res. 2015, 59, 106–128. [Google Scholar] [CrossRef] [PubMed]
- Dejam, A.; Hunter, C.J.; Schechter, A.N.; Gladwin, M.T. Emerging role of nitrite in human biology. Blood Cells Mol. Dis. 2004, 32, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.J.; Winyard, P.; Vanhatalo, A.; Blackwell, J.R.; Dimenna, F.J.; Wilkerson, D.P.; Tarr, J.; Benjamin, N.; Jones, A.M. Dietary nitrate supplementation reduces the O2 cost of low-intensity exercise and enhances tolerance to high-intensity exercise in humans. J. Appl. Physiol. 2009, 107, 1144–1155. [Google Scholar] [CrossRef] [PubMed]
- Larsen, F.J.; Weitzberg, E.; Lundberg, J.O.; Ekblom, B. Dietary nitrate reduces maximal oxygen consumption while maintaining work performance in maximal exercise. Free Radic Biol. Med. 2010, 48, 342–347. [Google Scholar] [CrossRef]
- Larsen, F.J.; Weitzberg, E.; Lundberg, J.O.; Ekblom, B. Effects of dietary nitrate on oxygen cost during exercise. Acta Physiol. 2007, 191, 59–66. [Google Scholar] [CrossRef]
- Wylie, L.J.; Kelly, J.; Bailey, S.J.; Blackwell, J.R.; Skiba, P.F.; Winyard, P.G.; Jeukendrup, A.E.; Vanhatalo, A.; Jones, A.M. Beetroot juice and exercise: Pharmacodynamic and dose-response relationships. J. Appl. Physiol. 2013, 115, 325–336. [Google Scholar] [CrossRef]
- Vermeer, I.T.; van Maanen, J.M. Nitrate exposure and the endogenous formation of carcinogenic nitrosamines in humans. Rev. Environ. Health 2001, 16, 105–116. [Google Scholar] [CrossRef]
- IARC monographs on the evaluation of carcinogenic risks to humans. Ingested nitrate and nitrite, and cyanobacterial peptide toxins. IARC Monogr. Eval. Carcinog. Risks Hum. 2010, 94, 1–412. [Google Scholar]
- Bogovski, P.; Bogovski, S. Animal Species in which N-nitroso compounds induce cancer. Int. J. Cancer 1981, 27, 471–474. [Google Scholar] [CrossRef] [PubMed]
- Bartsch, H. N-nitroso compounds and human cancer: Where do we stand? IARC Sci. Publ. 1991, 105, 1–10. [Google Scholar]
- Vermeer, I.T.; Pachen, D.M.; Dallinga, J.W.; Kleinjans, J.C.; van Maanen, J.M. Volatile N-nitrosamine formation after intake of nitrate at the ADI level in combination with an amine-rich diet. Environ. Health Perspect. 1998, 106, 459–463. [Google Scholar] [CrossRef] [PubMed]
- Maughan, R.J.; Burke, L.M.; Dvorak, J.; Larson-Meyer, D.E.; Peeling, P.; Phillips, S.M.; Rawson, E.S.; Walsh, N.P.; Garthe, I.; Geyer, H.; et al. IOC consensus statement: Dietary supplements and the high-performance athlete. Br. J. Sports Med. 2018, 52, 439–455. [Google Scholar] [CrossRef]
- Dejam, A.; Hunter, C.J.; Gladwin, M.T. Effects of dietary nitrate on blood pressure. N. Engl. J. Med. 2007, 356, 1590. [Google Scholar]
- Hohensinn, B.; Haselgrubler, R.; Muller, U.; Stadlbauer, V.; Lanzerstorfer, P.; Lirk, G.; Hoglinger, O.; Weghuber, J. Sustaining elevated levels of nitrite in the oral cavity through consumption of nitrate-rich beetroot juice in young healthy adults reduces salivary pH. Nitric Oxide Biol. Chem. 2016, 60, 10–15. [Google Scholar] [CrossRef] [Green Version]
- Henrohn, D.; Bjorkstrand, K.; Lundberg, J.O.; Granstam, S.O.; Baron, T.; Ingimarsdottir, I.J.; Hedenstrom, H.; Malinovschi, A.; Wernroth, M.L.; Jansson, M.; et al. Effects of Oral Supplementation with Nitrate-Rich Beetroot Juice in Patients with Pulmonary Arterial Hypertension-Results from BEET-PAH, an Exploratory Randomized, Double-Blind, Placebo-Controlled, Crossover Study. J. Card. Fail. 2018, 24, 640–653. [Google Scholar] [CrossRef]
- Magee, P.N.; Barnes, J.M. The production of malignant primary hepatic tumours in the rat by feeding dimethylnitrosamine. Br. J. Cancer 1956, 10, 114–122. [Google Scholar] [CrossRef]
- Sugimura, T.; Fujimura, S.; Baba, T. Tumor production in the glandular stomach and alimentary tract of the rat by N-methyl-N’-nitro-N-nitrosoguanidine. Cancer Res. 1970, 30, 455–465. [Google Scholar]
- Joosen, A.M.; Kuhnle, G.G.; Aspinall, S.M.; Barrow, T.M.; Lecommandeur, E.; Azqueta, A.; Collins, A.R.; Bingham, S.A. Effect of processed and red meat on endogenous nitrosation and DNA damage. Carcinogenesis. 2009, 30, 1402–1407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartholomew, B.; Hill, M.J. The pharmacology of dietary nitrate and the origin of urinary nitrate. Food Chem. Toxicol. 1984, 22, 789–795. [Google Scholar] [CrossRef]
- Leach, S.; Packer, P.; Hill, M. Salivary and urinary nitrate as measures of nitrate intake. Biochem. Soc. Trans. 1987, 15, 911–912. [Google Scholar] [CrossRef] [Green Version]
- Hill, M.J.; Hawksworth, G.; Tattersall, G. Bacteria, nitrosamines and cancer of the stomach. Br. J. Cancer 1973, 28, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.M. Stomach cancer mortality in Worksop and other Nottinghamshire mining towns. Br. J. Cancer 1980, 41, 438–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Roos, A.J.; Ward, M.H.; Lynch, C.F.; Cantor, K.P. Nitrate in public water supplies and the risk of colon and rectum cancers. Epidemiology 2003, 14, 640–649. [Google Scholar] [CrossRef]
- Vermeer, I.T.; Moonen, E.J.; Dallinga, J.W.; Kleinjans, J.C.; van Maanen, J.M. Effect of ascorbic acid and green tea on endogenous formation of N-nitrosodimethylamine and N-nitrosopiperidine in humans. Mutat. Res. 1999, 16, 353–361. [Google Scholar] [CrossRef]
- WHO Guidelines Approved by the Guidelines Review Committee. Guidelines for Drinking-Water Quality, 4th ed.; Incorporating the First Addendum; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Loh, Y.H.; Jakszyn, P.; Luben, R.N.; Mulligan, A.A.; Mitrou, P.N.; Khaw, K.T. N-Nitroso compounds and cancer incidence: The European Prospective Investigation into Cancer and Nutrition (EPIC)-Norfolk Study. Am. J. Clin. Nutr. 2011, 93, 1053–1061. [Google Scholar] [CrossRef]
- Keszei, A.P.; Goldbohm, R.A.; Schouten, L.J.; Jakszyn, P.; van den Brandt, P.A. Dietary N-nitroso compounds, endogenous nitrosation, and the risk of esophageal and gastric cancer subtypes in the Netherlands Cohort Study. Am. J. Clin. Nutr. 2013, 97, 135–146. [Google Scholar] [CrossRef]
- Larsson, S.C.; Bergkvist, L.; Wolk, A. Processed meat consumption, dietary nitrosamines and stomach cancer risk in a cohort of Swedish women. Int. J. Cancer 2006, 119, 915–919. [Google Scholar] [CrossRef] [Green Version]
- Knekt, P.; Jarvinen, R.; Dich, J.; Hakulinen, T. Risk of colorectal and other gastro-intestinal cancers after exposure to nitrate, nitrite and N-nitroso compounds: A follow-up study. Int. J. Cancer 1999, 80, 852–856. [Google Scholar] [CrossRef]
- Helser, M.A.; Hotchkiss, J.H.; Roe, D.A. Temporal influence of ascorbic acid dose on the endogenous formation of N-nitrosoproline and N-nitrosothiazolidine-4-carboxylic acid in humans. J. Nutr. Biochem. 1991, 2, 268–273. [Google Scholar] [CrossRef]
- Mirvish, S.S. Experimental evidence for inhibition of N-nitroso compound formation as a factor in the negative correlation between vitamin C consumption and the incidence of certain cancers. Cancer Res. 1994, 54, 1948s–1951s. [Google Scholar]
- Webb, A.J.; Patel, N.; Loukogeorgakis, S.; Okorie, M.; Aboud, Z.; Misra, S.; Rashid, R.; Miall, P.; Deanfield, J.; Benjamin, N. Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension 2008, 51, 784–790. [Google Scholar] [CrossRef]
- Kuhnle, G.G.; Story, G.W.; Reda, T.; Mani, A.R.; Moore, K.P.; Lunn, J.C.; Bingham, S.A. Diet-induced endogenous formation of nitroso compounds in the GI tract. Free Radic. Biol. Med. 2007, 43, 1040–1047. [Google Scholar] [CrossRef] [PubMed]
- Pisoschi, A.M.; Danet, A.F.; Kalinowski, S. Ascorbic Acid determination in commercial fruit juice samples by cyclic voltammetry. J. Autom. Methods Manag. Chem. 2008, 2008, 937651. [Google Scholar] [CrossRef] [PubMed]
- Şerban, M.; Câmpeanu, G.; Ionescu, E. Metode de Laborator în Biochimia Animală; Editura Didactica si Pedagogica: Bucuresti, Romania, 1993; p. 250. [Google Scholar]
- Papuc, C.; Pop, A.; Şerban, M. Metode Analitice in Biochimia Veterinara; Editura Printech: Bucuresti, Romania, 2001. [Google Scholar]
- Slot, C. Plasma creatinine determination. A new and specific Jaffe reaction method. Scand J. Clin. Lab. Investig. 1965, 17, 381–387. [Google Scholar] [CrossRef] [PubMed]




| All Subjects (n = 29) | BRJ + Vit C Group (n = 13) | BRJ Group (n = 16) | |
|---|---|---|---|
| Females/males | 16/13 | 9/4 | 7/9 |
| Age (y) | 21 ± 0.3 | 21 ± 0.6 | 21 ± 0.3 |
| BMI day 1 (kg/m2) | 23.0 ± 0.4 | 22.9 ± 0.6 | 23.2 ± 2.7 |
| BMI day 8 (kg/m2) | 23.0 ± 0.5 | 23.0 ± 0.5 | 23.0 ± 0.7 |
| All Subjects (n = 29) 1 | p-Values 2 | |||||
|---|---|---|---|---|---|---|
| Day 1 (baseline) | Day 2 | Day 8 | Day 1–2 | Day 1–8 | Day 2–8 | |
| Systolic BP (mmHg) | 111 (106–115) | NA | 112 (108–116) | NA 3 | NS 3 | NA 3 |
| Diastolic BP (mmHg) | 65 (62–68) | NA | 63 (62–68) | NA 3 | NS 3 | NA 3 |
| ATNC/creatinine (nmol/mmol) | 5 (<1; 9) | 47 (23; 71) | 104 (0.080; 0.128) | <0.0001 | <0.0001 | <0.01 |
| Nitrate/creatinine (µmol/mmol) | 0.708 (0.416; 1000) | 17.88 (14.75; 21.01) | 18.89 (15.75; 22.04) | <0.0001 | <0.001 | NS |
| Nitrite/creatinine (nmol/mmol) | 4 (0; 9) | 8 (0; 24) | 21 (12; 29) | NS | <0.001 | <0.001 |
| Vitamin C/creatinine (mmol/mmol) | 0.35 (0.23; 0.47) | 0.59 (0.46; 0.71) | 0.50 (0.39; 0.60) | <0.001 | <0.05 | NS |
| BRJ + Vit C Group (n = 13) 1 | p-Values *1 | BRJ Group (n = 16) 1 | p-Values 2 | p-Values Groups Compared 3 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 1 (baseline) | Day 2 | Day 8 | Day 1–2 | Day 1–8 | Day 2–8 | Day 1 (baseline) | Day 2 | Day 8 | Day 1–2 | Day 1–8 | Day 2–8 | Day 2 | Day 8 | |
| SBP (mmHg) 1*3 | 107(101; 114) | NA 2 | 109 (102; 116) | NA | NS 3 | NA | 113 (107; 119) | N/A | 114 (108;119) | NA 4 | NS 4 | NA 4 | NA 5 | NS 5 |
| DBP (mmHg) *3 | 64 (58; 70) | NA | 62 (58; 66) | NA | NS | NA | 66 (61; 70) | N/A | 65 (60; 70) | NA 4 | NS 4 | NA 4 | NA 5 | NS 5 |
| ATNC/creatinine (nmol/mmol) | 3 (0; 10) | 16 (6; 27) | 81 (50; 112) | NS | <0.01 | <0.01 | 6 (0; 13) | 72 (31; 113) | 123 (86; 159) | <0.01 | <0.0001 | <0.05 | <0.01 | NS |
| Nitrate/creatinine (µmol/mmol) | 0.56 (0.17; 0.95) | 17.61 (12.13; 23.09) | 18.19 (13.71; 22.66) | <0.001 | <0.001 | NS | 0.82 (0.37; 1.28) | 18.11 (13.96; 22.25) | 19.47 (14.61; 24.32) | <0.0001 | <0.0001 | NS | NS | NS |
| Nitrite/creatinine (nmol/mmol) | 6 (0; 17) | 0 (0; 0) | 25 (11; 39) | NS | <0.05 | <0.01 | 2 (0; 4) | 15 (14; 44) | 17 (5; 30) | NS | <0.05 | <0.05 | NS | NS |
| Vitamin C/creatinine (mmol/mmol) | 0.42 (0.17; 0.68) | 0.78 (0.56; 0.99) | 0.68 (0.52; 0.84) | <0.01 | NS | NS | 0.29 (0.20; 0.39) | 0.43 (0.30;0.56) | 0.35 (0.24; 0.46) | NS | NS | NS | NS | <0.05 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berends, J.E.; van den Berg, L.M.M.; Guggeis, M.A.; Henckens, N.F.T.; Hossein, I.J.; de Joode, M.E.J.R.; Zamani, H.; van Pelt, K.A.A.J.; Beelen, N.A.; Kuhnle, G.G.; et al. Consumption of Nitrate-Rich Beetroot Juice with or without Vitamin C Supplementation Increases the Excretion of Urinary Nitrate, Nitrite, and N-nitroso Compounds in Humans. Int. J. Mol. Sci. 2019, 20, 2277. https://doi.org/10.3390/ijms20092277
Berends JE, van den Berg LMM, Guggeis MA, Henckens NFT, Hossein IJ, de Joode MEJR, Zamani H, van Pelt KAAJ, Beelen NA, Kuhnle GG, et al. Consumption of Nitrate-Rich Beetroot Juice with or without Vitamin C Supplementation Increases the Excretion of Urinary Nitrate, Nitrite, and N-nitroso Compounds in Humans. International Journal of Molecular Sciences. 2019; 20(9):2277. https://doi.org/10.3390/ijms20092277
Chicago/Turabian StyleBerends, Julia E., Lauri M.M. van den Berg, Martina A. Guggeis, Nikki F.T. Henckens, Israt J. Hossein, Minke E.J.R. de Joode, Hossy Zamani, Kirsten A.A.J. van Pelt, Nicky A. Beelen, Gunter G. Kuhnle, and et al. 2019. "Consumption of Nitrate-Rich Beetroot Juice with or without Vitamin C Supplementation Increases the Excretion of Urinary Nitrate, Nitrite, and N-nitroso Compounds in Humans" International Journal of Molecular Sciences 20, no. 9: 2277. https://doi.org/10.3390/ijms20092277
APA StyleBerends, J. E., van den Berg, L. M. M., Guggeis, M. A., Henckens, N. F. T., Hossein, I. J., de Joode, M. E. J. R., Zamani, H., van Pelt, K. A. A. J., Beelen, N. A., Kuhnle, G. G., de Kok, T. M. C. M., & Van Breda, S. G. J. (2019). Consumption of Nitrate-Rich Beetroot Juice with or without Vitamin C Supplementation Increases the Excretion of Urinary Nitrate, Nitrite, and N-nitroso Compounds in Humans. International Journal of Molecular Sciences, 20(9), 2277. https://doi.org/10.3390/ijms20092277