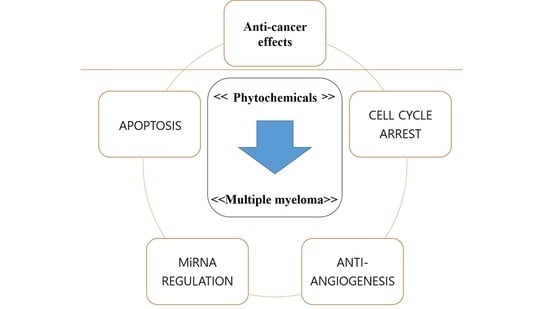
Anticancer Activity and Underlying Mechanism of Phytochemicals against Multiple Myeloma
Abstract
:1. Introduction
1.1. Current Drug Therapies for MM—And Their Limitations
1.2. Phytochemicals: A Possible Solution in Overcoming the Limitations of Contemporary Therapies?
2. Phytochemicals and MM
2.1. Anti-MM Effects of Natural Products via Intrinsic/Extrinsic Pathways of Apoptosis
2.1.1. Natural Products Induce Intrinsic Apoptosis
2.1.2. Phytochemicals Mediated through Extrinsic Pathways
2.1.3. Phytochemicals Mediated through Both Intrinsic and Extrinsic Pathways
2.2. Anti-MM Effects of Natural Products via Cell Cycle Arrest
2.3. Anti-MM Effects of Natural Products via Antiangiogenesis
2.4. Anti-MM Effects of Natural Products via miRNA Regulation
2.5. Clinical Trials of Natural Products on MM
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| MM | Multiple myeloma |
| IMiDs | immunomodulatory drugs |
| PIs | proteasome inhibitors |
| IMWG | International Multiple Myeloma Working Group |
| CRS | cytokine release syndrome |
| FADD | Fas-associated death domain |
| ROS | Reactive oxygen species |
| HDACs | histone deacetylases |
| HATs | histone acetyltransferases |
| TMP | tetramethylpyrazine |
| CK | Compound K |
| SB | Scutellaria baicalensis |
| TQ | Thymoquinone |
| EGCG | Epigallocatechin-3-gallate |
| DAPK2 | death-associated protein kinase 2 |
| VEGF | vascular endothelial growth factor |
| PDGF | platelet-derived growth factor |
| FGF | fibroblast growth factor |
| EGF | epidermal growth factor |
| TGFβ | transforming growth factor beta |
| MMPs | matrix metalloproteinase’s |
| TNF | tumor necrosis factor |
| Ang-1 | angiopoietins |
| uPAR | urokinase receptor |
| BM | bone marrow |
| BMM | bone marrow microenvironment |
| PCDC4 | programmed cell death 4 |
| GR | glucocorticoid receptors |
| ABM | Agaricus blazei Murrill |
| PBMCs | peripheral blood mononuclear cells |
References
- Agarwal, A.; Ghobrial, I.M.; Ghobrial, I.; Ghobrial, I.M. Monoclonal gammopathy of undetermined significance and Smoldering Multiple Myeloma: A review of the current understanding of epidemiology, biology, risk stratification and management of myeloma precursor disease. Clin. Cancer Res. 2013, 19, 985–994. [Google Scholar] [CrossRef] [PubMed]
- Kyle, R.A.; Gertz, M.A.; Witzig, T.E.; Lust, J.A.; Lacy, M.Q.; Dispenzieri, A.; Fonseca, R.; Rajkumar, S.V.; Offord, J.R.; Larson, D.R. In Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin. Proc. 2003, 78, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, A.; Tu, Y.; Fady, C.; Vescio, R.; Berenson, J. Interleukin-6 Inhibits Apoptosis of Malignant Plasma Cells. Cell. Immunol. 1995, 162, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Neben, K.; Moehler, T.; Benner, A.; Kraemer, A.; Egerer, G.; Ho, A.D.; Goldschmidt, H. Dose-dependent effect of thalidomide on overall survival in relapsed multiple myeloma. Clin. Cancer Res. 2002, 8, 3377–3382. [Google Scholar]
- Hus, M.; Dmoszynska, A.; Soroka-Wojtaszko, M.; Jawniak, D.; Legiec, W.; Ciepnuch, H.; Hellmann, A.; Wolska-Smolen, T.; Skotnicki, A.; Manko, J. Thalidomide treatment of resistant or relapsed multiple myeloma patients. Haematologica 2001, 86, 404–408. [Google Scholar]
- Wu, K.L.; Helgason, H.H.; van der Holt, B.; Wijermans, P.W.; Lokhorst, H.M.; Smit, W.; Sonneveld, P. Analysis of efficacy and toxicity of thalidomide in 122 patients with multiple myeloma: Response of soft-tissue plasmacytomas. Leukemia 2005, 19, 143–144. [Google Scholar] [CrossRef]
- Hideshima, T.; Chauhan, D.; Shima, Y.; Raje, N.; E Davies, F.; Tai, Y.T.; Treon, S.P.; Lin, B.; Schlossman, R.L.; Richardson, P.; et al. Thalidomide and its analogs overcome drug resistance of human multiple myeloma cells to conventional therapy. Blood 2000, 96, 2943–2950. [Google Scholar]
- Palumbo, A.; Giaccone, L.; Bertola, A.; Pregno, P.; Bringhen, S.; Rus, C.; Triolo, S.; Gallo, E.; Pileri, A.; Boccadoro, M. Low-dose thalidomide plus dexamethasone is an effective salvage therapy for advanced myeloma. Haematologica 2001, 86, 399–403. [Google Scholar]
- Anagnostopoulos, A.; Weber, D.; Rankin, K.; Delasalle, K.; Alexanian, R. Thalidomide and dexamethasone for resistant multiple myeloma. Br. J. Haematol. 2003, 121, 768–777. [Google Scholar] [CrossRef]
- Rajkumar, S.V.; Rosiñol, L.; Hussein, M.; Catalano, J.; Jedrzejczak, W.; Lucy, L.; Olesnyckyj, M.; Yu, Z.; Knight, R.; Zeldis, J.B.; et al. Multicenter, Randomized, Double-Blind, Placebo-Controlled Study of Thalidomide Plus Dexamethasone Compared with Dexamethasone as Initial Therapy for Newly Diagnosed Multiple Myeloma. J. Clin. Oncol. 2008, 26, 2171–2177. [Google Scholar] [CrossRef]
- Ghobrial, I.M.; Rajkumar, S.V. Management of thalidomide toxicity. J. Support. Oncol. 2003, 1, 194. [Google Scholar]
- Weber, D.M.; Chen, C.; Niesvizky, R.; Wang, M.; Belch, A.; Stadtmauer, E.A.; Siegel, D.; Borrello, I.; Rajkumar, S.V.; Chanan-Khan, A.A.; et al. Lenalidomide plus Dexamethasone for Relapsed Multiple Myeloma in North America. N. Engl. J. Med. 2007, 357, 2133–2142. [Google Scholar] [CrossRef]
- Adams, J. The proteasome: structure, function, and role in the cell. Cancer Treat. Rev. 2003, 29, 3–9. [Google Scholar] [CrossRef]
- Orlowski, R.Z. Phase I Trial of the Proteasome Inhibitor PS-341 in Patients with Refractory Hematologic Malignancies. J. Clin. Oncol. 2002, 20, 4420–4427. [Google Scholar] [CrossRef]
- Richardson, P.G.; Barlogie, B.; Berenson, J.; Singhal, S.; Jagannath, S.; Irwin, D.; Rajkumar, S.V.; Srkalovic, G.; Alsina, M.; Alexanian, R.; et al. A Phase 2 Study of Bortezomib in Relapsed, Refractory Myeloma. N. Engl. J. Med. 2003, 348, 2609–2617. [Google Scholar] [CrossRef]
- Richardson, P.G.; Sonneveld, P.; Schuster, M.; Irwin, D.; Stadtmauer, E.; Facon, T.; Harousseau, J.-L.; Ben-Yehuda, D.; Lonial, S.; Goldschmidt, H.; et al. Extended follow-up of a phase 3 trial in relapsed multiple myeloma: final time-to-event results of the APEX trial. Blood 2007, 110, 3557–3560. [Google Scholar] [CrossRef]
- Richardson, P.G.; Hideshima, T.; Anderson, K.C. Bortezomib (PS-341): A Novel, First-in-Class Proteasome Inhibitor for the Treatment of Multiple Myeloma and Other Cancers. Cancer Control 2003, 10, 361–369. [Google Scholar] [CrossRef]
- Adams, G.P.; Weiner, L.M. Monoclonal antibody therapy of cancer. Nat. Biotechnol. 2005, 23, 1147–1157. [Google Scholar] [CrossRef]
- De Weers, M.; Tai, Y.-T.; van der Veer, M.S.; Bakker, J.M.; Vink, T.; Jacobs, D.C.; Oomen, L.A.; Peipp, M.; Valerius, T.; Slootstra, J.W. Daratumumab, a novel therapeutic human cd38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J. Immunol. 2011, 186, 1840–1848. [Google Scholar] [CrossRef]
- Presta, L.G. Engineering of therapeutic antibodies to minimize immunogenicity and optimize function. Adv. Drug Deliv. Rev. 2006, 58, 640–656. [Google Scholar] [CrossRef]
- Cox, L.; Platts-Mills, T.A.; Finegold, I.; Schwartz, L.B.; Simons, F.E.R.; Wallace, D.V. American Academy of Allergy, Asthma & Immunology/American College of Allergy, Asthma and Immunology Joint Task Force Report on omalizumab-associated anaphylaxis. J. Allergy Clin. Immunol. 2007, 120, 1373–1377. [Google Scholar]
- Corren, J.; Casale, T.B.; Lanier, B.; Buhl, R.; Holgate, S.; Jimenez, P. Safety and tolerability of omalizumab. Clin. Exp. Allergy 2009, 39, 788–797. [Google Scholar] [CrossRef]
- Todd, D.J.; Helfgott, S.M. Serum sickness following treatment with rituximab. J. Rheumatol. 2007, 34, 430–433. [Google Scholar]
- Gaston, R.S.; Deierhoi, M.H.; Patterson, T.; Prasthofer, E.; Julian, B.A.; Barber, W.H.; Laskow, D.A.; Diethelm, A.G.; Curtis, J.J. OKT3 first-dose reaction: Association with T cell subsets and cytokine release. Kidney Int. 1991, 39, 141–148. [Google Scholar] [CrossRef]
- Wing, M.G.; Waldmann, H.; Isaacs, J.; A Compston, D.; Hale, G. Ex-vivo whole blood cultures for predicting cytokine-release syndrome: dependence on target antigen and antibody isotype. Ther. Immunol. 1995, 2, 183–190. [Google Scholar]
- Wing, M.G.; Moreau, T.; Greenwood, J.; Smith, R.M.; Hale, G.; Isaacs, J.; Waldmann, H.; Lachmann, P.J.; Compston, A. Mechanism of first-dose cytokine-release syndrome by CAMPATH 1-H: involvement of CD16 (FcgammaRIII) and CD11a/CD18 (LFA-1) on NK cells. J. Clin. Investig. 1996, 98, 2819–2826. [Google Scholar] [CrossRef]
- Dobson, P.D.; Patel, Y.; Kell, D.B. ‘Metabolite-likeness’ as a criterion in the design and selection of pharmaceutical drug libraries. Drug Discov. Today 2009, 14, 31–40. [Google Scholar] [CrossRef]
- Ganesan, A. The impact of natural products upon modern drug discovery. Chem. Biol. 2008, 12, 306–317. [Google Scholar] [CrossRef]
- Schenone, M.; Dancik, V.; Wagner, B.K.; A Clemons, P. Target identification and mechanism of action in chemical biology and drug discovery. Nat. Methods 2013, 9, 232–240. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef]
- Raimondi, L.; De Luca, A.; Giavaresi, G.; Barone, A.; Tagliaferri, P.; Tassone, P.; Amodio, N. Impact of natural dietary agents on multiple myeloma prevention and treatment: molecular insights and potential for clinical translation. Med. Chem. 2018, 25, 1. [Google Scholar] [CrossRef]
- Renehan, A.G.; Booth, C.; Potten, C.S.; Renehan, A.G.; Renehan, A. What is apoptosis, and why is it important? BMJ 2001, 322, 1536–1538. [Google Scholar] [CrossRef]
- Adams, J.M. The Bcl-2 Protein Family: Arbiters of Cell Survival. Science 1998, 281, 1322–1326. [Google Scholar] [CrossRef]
- Lamorte, D.; Faraone, I.; Laurenzana, I.; Milella, L.; Trino, S.; de Luca, L.; del Vecchio, L.; Armentano, M.F.; Sinisgalli, C.; Chiummiento, L.; et al. Future in the Past: Azorella glabra Wedd. as a Source of New Natural Compounds with Antiproliferative and Cytotoxic Activity on Multiple Myeloma Cells. Int. J. Mol. Sci. 2018, 19, 3348. [Google Scholar] [CrossRef]
- Hu, H.-y.; Li, K.-p.; Wang, X.-j.; Liu, Y.; Lu, Z.-g.; Dong, R.-h.; Guo, H.-b.; Zhang, M.-x. Set9, NF-κB, and microRNA-21 mediate berberine-induced apoptosis of human multiple myeloma cells. Acta Pharmacol. Sin. 2013, 34, 157. [Google Scholar] [CrossRef]
- Yang, X.-J.; Seto, E. HATs and HDACs: From structure, function and regulation to novel strategies for therapy and prevention. Oncogene 2007, 26, 5310–5318. [Google Scholar] [CrossRef]
- Kim, S.-H.; Sohn, E.J.; Jung, J.H.; Lee, M.H.; Kim, B.; Jeong, S.-J.; Kim, S.-H. Brazil in Induces Apoptosis and G2/M Arrest via Inactivation of Histone Deacetylase in Multiple Myeloma U266 Cells. J. Agric. Food Chem. 2012, 60, 9882–9889. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Nair, A.S.; Sung, B.; Pandey, M.K.; Aggarwal, B.B. Boswellic acid blocks signal transducers and activators of transcription 3 signaling, proliferation, and survival of multiple myeloma via the protein tyrosine phosphatase SHP-1. Mol. Cancer Res. 2009, 7, 118–128. [Google Scholar] [CrossRef]
- Sun, X.; Liao, W.; Wang, J.; Wang, P.; Gao, H.; Wang, M.; Xu, C.; Zhong, Y.; Ding, Y. CSTMP induces apoptosis and mitochondrial dysfunction in human myeloma RPMI8226 cells via CHOP-dependent endoplasmic reticulum stress. Biomed. Pharmacother. 2016, 83, 776–784. [Google Scholar] [CrossRef]
- Hatcher, H.; Planalp, R.; Cho, J.; Torti, F.M.; Torti, S.V. Curcumin: From ancient medicine to current clinical trials. Cell. Mol. Life Sci. 2008, 65, 1631–1652. [Google Scholar] [CrossRef]
- Muto, A.; Hori, M.; Sasaki, Y.; Saitoh, A.; Yasuda, I.; Maekawa, T.; Uchida, T.; Asakura, K.; Nakazato, T.; Kaneda, T.; et al. Emodin has a cytotoxic activity against human multiple myeloma as a Janus-activated kinase 2 inhibitor. Mol. Cancer Ther. 2007, 6, 987–994. [Google Scholar] [CrossRef]
- Lee, J.C.; Ahn, K.S.; Jeong, S.-J.; Jung, J.H.; Kwon, T.-R.; Rhee, Y.-H.; Kim, S.-H.; Kim, S.-Y.; Yoon, H.-J.; Zhu, S.; et al. Signal transducer and activator of transcription 3 pathway mediates genipin-induced apoptosis in U266 multiple myeloma cells. J. Cell. Biochem. 2011, 112, 1552–1562. [Google Scholar] [CrossRef]
- Park, S.; Lee, H.-J.; Jeong, S.-J.; Song, H.S.; Kim, M.; Lee, H.-J.; Lee, E.-O.; Kim, D.-H.; Ahn, K.S.; Kim, S.-H. Inhibition of JAK1/STAT3 signaling mediates compound K-induced apoptosis in human multiple myeloma U266 cells. Food Chem. Toxicol. 2011, 49, 1367–1372. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, S.; Wu, J.; Yu, K.; Zhang, Y.; Yin, L.; Bi, L. Matrine induces apoptosis of human multiple myeloma cells via activation of the mitochondrial pathway. Leuk. Lymphoma 2010, 51, 1337–1346. [Google Scholar]
- Ikezoe, T.; Yang, Y.; Bandobashi, K.; Saito, T.; Takemoto, S.; Machida, H.; Togitani, K.; Koeffler, H.P.; Taguchi, H. Oridonin, a diterpenoid purified from Rabdosia rubescens, inhibits the proliferation of cells from lymphoid malignancies in association with blockade of the NF-κB signal pathways. Mol. Cancer Ther. 2005, 4, 578–586. [Google Scholar] [CrossRef]
- Kiraz, Y.; Neergheen-Bhujun, V.S.; Rummun, N.; Baran, Y. Apoptotic effects of non-edible parts of Punica granatum on human multiple myeloma cells. Tumor Biol. 2016, 37, 1803–1815. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Sethi, G.; Vadhan-Raj, S.; Bueso-Ramos, C.; Takada, Y.; Gaur, U.; Nair, A.S.; Shishodia, S.; Aggarwal, B.B. Resveratrol inhibits proliferation, induces apoptosis, and overcomes chemoresistance through down-regulation of STAT3 and nuclear factor-κB–regulated antiapoptotic and cell survival gene products in human multiple myeloma cells. Blood 2007, 109, 2293–2302. [Google Scholar] [CrossRef]
- Kumagai, T.; Müller, C.I.; Desmond, J.C.; Imai, Y.; Heber, D.; Koeffler, H.P. Scutellaria baicalensis, a herbal medicine: Anti-proliferative and apoptotic activity against acute lymphocytic leukemia, lymphoma and myeloma cell lines. Leuk. Res. 2007, 31, 523–530. [Google Scholar] [CrossRef]
- Rao, P.S.; Ramanadham, M.; Prasad, M.N.V. Anti-proliferative and cytotoxic effects of Strychnos nux-vomica root extract on human multiple myeloma cell line—RPMI 8226. Food Chem. Toxicol. 2009, 47, 283–288. [Google Scholar] [CrossRef]
- Badr, G.; Lefevre, E.A.; Mohany, M. Thymoquinone Inhibits the CXCL12-Induced Chemotaxis of Multiple Myeloma Cells and Increases Their Susceptibility to Fas-Mediated Apoptosis. PLoS ONE 2011, 6, e23741. [Google Scholar] [CrossRef]
- Mitsiades, C.S.; Ocio, E.M.; Pandiella, A.; Maiso, P.; Gajate, C.; Garayoa, M.; Vilanova, D.; Montero, J.C.; Mitsiades, N.; McMullan, C.J.; et al. Aplidin, a Marine Organism-Derived Compound with Potent Antimyeloma Activity In Vitro and In Vivo. Cancer Res. 2008, 68, 5216–5225. [Google Scholar] [CrossRef]
- Sagawa, M.; Nakazato, T.; Uchida, H.; Ikeda, Y.; Kizaki, M. Cantharidin induces apoptosis of human multiple myeloma cells via inhibition of the JAK/STAT pathway. Cancer Sci. 2008, 99, 1820–1826. [Google Scholar] [CrossRef]
- Sato, M.; Sagawa, M.; Nakazato, T.; Ikeda, Y.; Kizaki, M. A natural peptide, dolastatin 15, induces G2/M cell cycle arrest and apoptosis of human multiple myeloma cells. Int. J. Oncol. 2007, 30, 1453–1459. [Google Scholar] [CrossRef]
- Shammas, M.A.; Neri, P.; Koley, H.; Batchu, R.B.; Bertheau, R.C.; Munshi, V.; Prabhala, R.; Fulciniti, M.; Tai, Y.T.; Treon, S.P.; et al. Specific killing of multiple myeloma cells by (−)-epigallocatechin-3-gallate extracted from green tea: Biologic activity and therapeutic implications. Blood 2006, 108, 2804–2810. [Google Scholar] [CrossRef]
- Pietenpol, J.; Stewart, Z. Cell cycle checkpoint signaling: Cell cycle arrest versus apoptosis. Toxicology 2002, 181, 475–481. [Google Scholar] [CrossRef]
- Blagosklonny, M.V.; Pardee, A.B. The Restriction Point of the Cell Cycle. Cell Cycle 2002, 1, 102–109. [Google Scholar] [CrossRef]
- Kastan, M.B.; Bartek, J. Cell-cycle checkpoints and cancer. Nature 2004, 432, 316. [Google Scholar] [CrossRef]
- Vermeulen, K.; van Bockstaele, D.R.; Berneman, Z.N. The cell cycle: a review of regulation, deregulation and therapeutic targets in cancer. Cell Prolif. 2003, 36, 131–149. [Google Scholar] [CrossRef]
- Kaufmann, W.K.; Paules, R.S. DNA damage and cell cycle checkpoints. FASEB J. 1996, 10, 238–247. [Google Scholar] [CrossRef]
- Zhao, F.; Chen, Y.; Li, R.; Liu, Y.; Wen, L.; Zhang, C. Triptolide alters histone H3K9 and H3K27 methylation state and induces G0/G1 arrest and caspase-dependent apoptosis in multiple myeloma in vitro. Toxicology 2010, 267, 70–79. [Google Scholar] [CrossRef]
- Rajabi, M.; Mousa, S.A. The Role of Angiogenesis in Cancer Treatment. Biomedicines 2017, 5, 34. [Google Scholar] [CrossRef]
- Ferrara, N.; Davis-Smyth, T. The Biology of Vascular Endothelial Growth Factor. Endocr. Rev. 1997, 18, 4–25. [Google Scholar] [CrossRef]
- Yadav, L.; Puri, N.; Rastogi, V.; Satpute, P.; Sharma, V. Tumour angiogenesis and angiogenic inhibitors: A review. J. Clin. Diagn. Res. 2015, 9, XE01. [Google Scholar] [CrossRef]
- Zheng, Y.; Sun, Y.; Yu, X.; Shao, Y.; Zhang, P.; Dai, G.; Fu, J. Angiogenesis in liquid tumors: An in-vitro assay for leukemic cell induced bone marrow angiogenesis. Adv. Heal. Mater. 2016, 5, 1014–1024. [Google Scholar] [CrossRef]
- Issa, M.E.; Berndt, S.; Carpentier, G.; Pezzuto, J.M.; Cuendet, M. Bruceantin inhibits multiple myeloma cancer stem cell proliferation. Cancer Biol. Ther. 2016, 17, 966–975. [Google Scholar] [CrossRef]
- Fu, R.; Chen, Y.; Wang, X.-P.; An, T.; Tao, L.; Zhou, Y.-X.; Huang, Y.-J.; Chen, B.-A.; Li, Z.-Y.; You, Q.-D. Wogonin inhibits multiple myeloma-stimulated angiogenesis via c-Myc/VHL/HIF-1α signaling axis. Oncotarget 2016, 7, 5715. [Google Scholar]
- Kim, S.-M.; Lee, J.H.; Sethi, G.; Kim, C.; Baek, S.H.; Nam, D.; Chung, W.-S.; Kim, S.-H.; Shim, B.S.; Ahn, K.S. Bergamottin, a natural furanocoumarin obtained from grapefruit juice induces chemosensitization and apoptosis through the inhibition of STAT3 signaling pathway in tumor cells. Cancer Lett. 2014, 354, 153–163. [Google Scholar] [CrossRef]
- Arbiser, J.L.; Kau, T.; Konar, M.; Narra, K.; Ramchandran, R.; Summers, S.A.; Vlahos, C.J.; Ye, K.; Perry, B.N.; Matter, W.; et al. Solenopsin, the alkaloidal component of the fire ant (Solenopsis invicta), is a naturally occurring inhibitor of phosphatidylinositol-3-kinase signaling and angiogenesis. Blood 2007, 109, 560–565. [Google Scholar] [CrossRef]
- Ikeda, N.E.A.; Novak, E.M.; Maria, D.A.; Velosa, A.S.; Pereira, R.M.S. Synthesis, characterization and biological evaluation of Rutin–zinc(II) flavonoid–metal complex. Chem. Interactions 2015, 239, 184–191. [Google Scholar] [CrossRef]
- Chen, H.; Shi, L.; Yang, X.; Li, S.; Guo, X.; Pan, L. Artesunate inhibiting angiogenesis induced by human myeloma RPMI8226 cells. Int. J. Hematol. 2010, 92, 587–597. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Ni Bong, I.P.; Ng, C.C.; Baharuddin, P.; Zakaria, Z. MicroRNA expression patterns and target prediction in multiple myeloma development and malignancy. Genes Genom. 2017, 39, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Manier, S.; Sacco, A.; Leleu, X.; Ghobrial, I.M.; Roccaro, A.M. Bone Marrow Microenvironment in Multiple Myeloma Progression. J. Biomed. Biotechnol. 2012, 2012, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Löffler, D.; Brocke-Heidrich, K.; Pfeifer, G.; Stocsits, C.; Hackermüller, J.; Kretzschmar, A.K.; Burger, R.; Gramatzki, M.; Blumert, C.; Bauer, K. Interleukin-6–dependent survival of multiple myeloma cells involves the STAT3-mediated induction of microRNA-21 through a highly conserved enhancer. Blood 2007, 110, 1330–1333. [Google Scholar] [CrossRef] [PubMed]
- Chi, J.; Ballabio, E.; Chen, X.-H.; Kušec, R.; Taylor, S.; Hay, D.; Tramonti, D.; Saunders, N.J.; Littlewood, T.; Pezzella, F.; et al. MicroRNA expression in multiple myeloma is associated with genetic subtype, isotype and survival. Biol. Direct 2011, 6, 23. [Google Scholar] [CrossRef] [PubMed]
- Pichiorri, F.; Suh, S.-S.; Ladetto, M.; Kuehl, M.; Palumbo, T.; Drandi, D.; Taccioli, C.; Zanesi, N.; Alder, H.; Hagan, J.P.; et al. MicroRNAs regulate critical genes associated with multiple myeloma pathogenesis. Proc. Natl. Acad. Sci. USA 2008, 105, 12885–12890. [Google Scholar] [CrossRef] [PubMed]
- Pichiorri, F.; Suh, S.-S.; Rocci, A.; de Luca, L.; Taccioli, C.; Santhanam, R.; Zhou, W.; Benson, D.M.; Hofmainster, C.; Alder, H. Downregulation of p53-inducible microRNAs 192, 194, and 215 impairs the p53/MDM2 autoregulatory loop in multiple myeloma development. Cancer Cell 2010, 18, 367–381. [Google Scholar] [CrossRef] [PubMed]
- Sax, J.K.; Fei, P.; Murphy, M.E.; Bernhard, E.; Korsmeyer, S.J.; El-Deiry, W.S. BID regulation by p53 contributes to chemosensitivity. Nat. Cell Biol. 2002, 4, 842–849. [Google Scholar] [CrossRef]
- Herrero, A.B.; Rojas, E.A.; Misiewicz-Krzeminska, I.; Krzeminski, P.; Gutiérrez, N.C.; Iwakuma, T. Molecular Mechanisms of p53 Deregulation in Cancer: An Overview in Multiple Myeloma. Int. J. Mol. Sci. 2016, 17, 2003. [Google Scholar] [CrossRef]
- Saki, N.; Abroun, S.; Hajizamani, S.; Rahim, F.; Shahjahani, M. Association of Chromosomal Translocation and miRNA Expression with The Pathogenesis of Multiple Myeloma. Cell J. 2014, 16, 99–110. [Google Scholar]
- Zhang, C.; Liu, J.; Wang, X.; Feng, Z. The regulation of the p53/MDM2 feedback loop by microRNAs. RNA Dis. 2015, 2, e502. [Google Scholar]
- Undi, R.B.; Kandi, R.; Gutti, R.K. MicroRNAs as Haematopoiesis Regulators. Adv. Hematol. 2013, 2013, 1–20. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, L.; Li, D.; Yin, Y.; Zhang, C.-Y.; Li, J.; Zhang, Y. Microvesicle-delivery miR-150 promotes tumorigenesis by up-regulating VEGF, and the neutralization of miR-150 attenuate tumor development. Protein Cell 2013, 4, 932–941. [Google Scholar] [CrossRef]
- Raimondi, L.; de Luca, A.; Morelli, E.; Giavaresi, G.; Tagliaferri, P.; Tassone, P.; Amodio, N. MicroRNAs: Novel Crossroads between Myeloma Cells and the Bone Marrow Microenvironment. BioMed Int. 2016, 2016, 1–12. [Google Scholar] [CrossRef]
- Amodio, N.; di Martino, M.T.; Foresta, U.; Leone, E.; Lionetti, M.; Leotta, M.; Gullà, A.M.; Pitari, M.R.; Conforti, F.; Rossi, M.; et al. miR-29b sensitizes multiple myeloma cells to bortezomib-induced apoptosis through the activation of a feedback loop with the transcription factor Sp1. Cell Death 2012, 3, e436. [Google Scholar] [CrossRef]
- Di Martino, M.T.; Leone, E.; Amodio, N.; Foresta, U.; Lionetti, M.; Pitari, M.R.; Cantafio, M.E.G.; Gullà, A.; Conforti, F.; Morelli, E.; et al. Synthetic miR-34a mimics as a novel therapeutic agent for Multiple Myeloma: In Vitro and in vivo evidence. Clin. Cancer Res. 2012, 18, 6260–6270. [Google Scholar] [CrossRef]
- Wu, S.; Yu, W.; Qu, X.; Wang, R.; Xu, J.; Zhang, Q.; Xu, J.; Li, J.; Chen, L. Argonaute 2 promotes myeloma angiogenesis via microRNA dysregulation. J. Hematol. Oncol. 2014, 7, 40. [Google Scholar] [CrossRef]
- Leotta, M.; Biamonte, L.; Raimondi, L.; Ronchetti, D.; Di Martino, M.T.; Botta, C.; Leone, E.; Pitari, M.R.; Neri, A.; Giordano, A.; et al. A p53-Dependent Tumor Suppressor Network Is Induced by Selective miR-125a-5p Inhibition in Multiple Myeloma Cells. J. Cell. Physiol. 2014, 229, 2106–2116. [Google Scholar] [CrossRef]
- Luo, X.; Gu, J.; Zhu, R.; Feng, M.; Zhu, X.; Li, Y.; Fei, J. Integrative analysis of differential miRNA and functional study of miR-21 by seed-targeting inhibition in multiple myeloma cells in response to berberine. BMC Syst. Biol. 2014, 8, 82. [Google Scholar] [CrossRef]
- Feng, R.; Shou, J.-W.; Zhao, Z.-X.; He, C.-Y.; Ma, C.; Huang, M.; Fu, J.; Tan, X.-S.; Li, X.-Y.; Wen, B.-Y.; et al. Transforming berberine into its intestine-absorbable form by the gut microbiota. Sci. Rep. 2015, 5, 12155. [Google Scholar] [CrossRef]
- Huang, X.; Yang, M.; Jin, J. Triptolide enhances the sensitivity of multiple myeloma cells to dexamethasone via microRNAs. Leuk. Lymphoma 2012, 53, 1188–1195. [Google Scholar] [CrossRef]
- Firenzuoli, F.; Gori, L.; Lombardo, G. The medicinal mushroom Agaricus blazei murrill: Review of literature and pharmaco-toxicological problems. Evid. Based Complement. Alternat. Med. 2008, 5, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Murakawa, K.; Fukunaga, K.; Tanouchi, M.; Hosokawa, M.; Hossain, Z.; Takahashi, K. Therapy of Myeloma In Vivo Using Marine Phospholipid in Combination with Agaricus blazei Murill as an Immune Respond Activator. J. Oleo Sci. 2007, 56, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Tangen, J.M.; Tierens, A.; Caers, J.; Binsfeld, M.; Olstad, O.K.; Troseid, A.M.; Wang, J.; Tjonnfjord, G.E.; Hetland, G. Immunomodulatory effects of the Agaricus blazei murrill-based mushroom extract and osan in patients with multiple myeloma undergoing high dose chemotherapy and autologous stem cell transplantation: A randomized, double blinded clinical study. Biomed. Res. Int. 2015, 2015, 718539. [Google Scholar] [CrossRef]
- Falardeau, P.; Champagne, P.; Poyet, P.; Hariton, C.; Dupont, É. Neovastat, a naturally occurring multifunctional antiangiogenic drug, in phase III clinical trials. Semin. Oncol. 2001, 28, 620–625. [Google Scholar] [CrossRef]
- Kim, C.; Kim, B. Anti-Cancer Natural Products and Their Bioactive Compounds Inducing ER Stress-Mediated Apoptosis: A Review. Nutrients 2018, 10, 1021. [Google Scholar] [CrossRef] [PubMed]
- Sook, S.H.; Lee, H.J.; Kim, J.H.; Sohn, E.J.; Jung, J.H.; Kim, B.; Kim, J.H.; Jeong, S.J.; Kim, S.H. Reactive oxygen species-mediated activation of AMP-activated protein kinase and c-Jun N-terminal kinase plays a critical role in beta-sitosterol-induced apoptosis in multiple myeloma U266 cells. Phytother. Res. 2014, 28, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Song, H.S.; Park, H.; Kim, B. Activation of ER stress-dependent miR-216b has a critical role in Salvia miltiorrhiza ethanol-extract-induced apoptosis in U266 and U937 cells. Int. J. Mol. Sci. 2018, 19, 1240. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.A.; Song, H.-S.; Kang, B.; Park, M.N.; Park, K.S.; Kim, S.-H.; Shim, B.-S.; Kim, B. miR-211 Plays a Critical Role in Cnidium officinale Makino Extract-Induced, ROS/ER Stress-Mediated Apoptosis in U937 and U266 Cells. Int. J. Mol. Sci. 2018, 19, 865. [Google Scholar] [CrossRef]
- Angtuaco, E.J.C.; Fassas, A.B.T.; Walker, R.; Sethi, R.; Barlogie, B. Multiple Myeloma: Clinical Review and Diagnostic Imaging. Radiology 2004, 231, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Hemaiswarya, S.; Doble, M. Potential synergism of natural products in the treatment of cancer. Phytother. Res. 2006, 20, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Amodio, N.; D’Aquila, P.; Passarino, G.; Tassone, P.; Bellizzi, D. Epigenetic modifications in multiple myeloma: Recent advances on the role of DNA and histone methylation. Expert Opin. Ther. Targets 2017, 21, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Amodio, N.; Stamato, M.A.; Juli, G.; Morelli, E.; Fulciniti, M.; Manzoni, M.; Taiana, E.; Agnelli, L.; Cantafio, M.E.G.; Romeo, E.; et al. Drugging the lncRNA MALAT1 via LNA gapmeR ASO inhibits gene expression of proteasome subunits and triggers anti-multiple myeloma activity. Leukemia 2018, 32, 1948–1957. [Google Scholar] [CrossRef] [PubMed]
- Amodio, N.; Raimondi, L.; Juli, G.; Stamato, M.A.; Caracciolo, D.; Tagliaferri, P.; Tassone, P. MALAT1: A druggable long non-coding RNA for targeted anti-cancer approaches. J. Hematol. Oncol. 2018, 11, 63. [Google Scholar] [CrossRef] [PubMed]
- Pitari, M.R.; Rossi, M.; Amodio, N.; Botta, C.; Morelli, E.; Federico, C.; Gullà, A.; Caracciolo, D.; di Martino, M.T.; Arbitrio, M.; et al. Inhibition of miR-21 restores RANKL/OPG ratio in multiple myeloma-derived bone marrow stromal cells and impairs the resorbing activity of mature osteoclasts. Oncotarget 2015, 6, 27343–27358. [Google Scholar] [CrossRef] [PubMed]
- Morelli, E.; Biamonte, L.; Federico, C.; Amodio, N.; di Martino, M.T.; Gallo Cantafio, M.E.; Manzoni, M.; Scionti, F.; Samur, M.K.; Gulla, A.; et al. Therapeutic vulnerability of multiple myeloma to miR17PTi, a first-in-class inhibitor of pri-miR-17-92. Blood 2018, 132, 1050–1063. [Google Scholar] [CrossRef] [PubMed]
- Moudi, M.; Go, R.; Yien, C.Y.S.; Nazre, M. Vinca alkaloids. Int. J. Prev. Med. 2013, 4, 1231. [Google Scholar]
- McGowan, J.V.; Chung, R.; Maulik, A.; Piotrowska, I.; Walker, J.M.; Yellon, D.M. Anthracycline Chemotherapy and Cardiotoxicity. Cardiovasc. Drugs Ther. 2017, 31, 63–75. [Google Scholar] [CrossRef]





| Source | Compound | Cell Line | Dose/Duration | Mechanism | References |
|---|---|---|---|---|---|
| Azorella glabra Wedd. (AG) | AG extract | RPMI8226, SKMM1, MM1S | 50 µg/mL; 24, 48 h | c-PARP, c-caspase-3 ↑ Bcl-2 ↓ | [34] |
| Coptis chinensis Franch. | Berberine | U266 | 0, 40, 80, 120, 160 μmol/L; 24 h | PUMA/caspase-3, caspase-9 ↑ Bcl-2 ↓ | [35] |
| Caesalpinia sappan (L.) Tod. | Brazilin | U266 | 60 μM; 0, 6, 12, 24 h | c-caspase-3, c-PARP ↑ Bcl-xL, HDACs ↓ | [36,37] |
| Boswellia serrata Spreng. | Boswellic acid | U266 | 50 μmol/L; 4 h | c-caspase-3, c-PARP ↑ survivin, bcl-xl, bcl-2, Mcl-1 ↓ | [38] |
| Ligusticum wallichii Franch. | Tetramethylpyrazine (TMP) | RPMI8226 | 0, 10, 75, 150, 300 μM; 48 h | c-caspase-3, 8, 9, Bax, Cyto c release, CHOP, cleaved caspase-12, GRP78, GRP94, p-PERK, p-eIF2a, IRE1a, ATF6 ↑ Bcl-2, Bcl-xL ↓ | [39] |
| Curcuma longa Linn | Curcumin | U266, RPMI 8226 | 10 μM; 24 h | c-caspase-3, -8, c-BID, Cyto c release ↑ | [40] |
| Rheum palmatum Linn | Emodin | U266, RPMI 8226, IM-9 | 1, 10, 20, 50, 100 μM/L; 24 h | c-caspase-3, -9 ↑ Mcl-1, JAK2, STAT3 ↓ | [41] |
| Gardenia jasminoides J.Ellis | Genipin | U266 | 100 μM; 0, 24, 48, 72 h | STAT3, c-Src, Bcl-2, Bcl-xL, survivin, cyclin D1, VEGF ↓ | [42] |
| Panax ginseng C.A.Mey. | Compound K (CK) | U266 | 0, 5, 10, 25, 50, 100 μM; 24 h | c-PARP, c-caspase-3 ↑ Bcl-xL, Bcl-2, surviving ↓ | [43] |
| Sophora flavescens Aiton | Matrine | U266, RPMI 8226 | 0.25, 0.5, 1.0, 1.5, 2.0, 3.0 g/L; 48 h | c-caspase-3, cyto c release, Bax ↑ Bcl-2, MMP ↓ | [44] |
| Rabdosia rubescens (Hemsl.) H.Hara | Oridonin | U266, RPMI8226 | 1, 2 μg/mL; 24 h | Mcl-1, Bcl-xL ↓ | [45] |
| Punica granatum L. | Pomegrante extract | U266 | P. granatum flower extracts: 1, 10, 50, 100 μg/mL; 48, 72 h, P. granatum stem and leaves extracts: 1, 10, 50, 100, 500 μg/mL; 48, 72 h, | MMP ↓ | [46] |
| Veratrum grandiflorum Loes | Resveratrol | U266, RPMI 8226 | 0, 15, 25, 30 μM; 24 h | Bax, c-caspase-3 ↑ cyclin D1, cIAP-2, XIAP, survivin, Bcl-2, Bcl-xL, Bfl-1/A1, TRAF2, AKT ↓ | [47] |
| Scutellaria baicalensis Georgi (SB) | SB extract | U266, NCI-H929 | 50 g/mL; 48 h | p27KIP1, Bax ↑ Bcl-2, Bcl-xL ↓ | [48] |
| Strychnos nux-vomica L. (SN) | SN root extract | RPMI 8226 | 11, 22, 44 mg/mL | cyto C release ↑ MMP ↓ | [49] |
| Source | Compound | Cell Line | Dose/Duration | Mechanism | References |
|---|---|---|---|---|---|
| Nigella sativa Linn | Thymoquinone | MDN, XG-2 | 10 µM; 24 h | CD95 ↑ | [50] |
| Source | Compound | Cell Line | Dose/Duration | Mechanism | References |
|---|---|---|---|---|---|
| Aplidium albicans | Alipidin | U266, MM.1S, MM.1R, U266-LR7 | 0, 1, 2, 5, 10, 20, 50, 100 nmol/L; 72 h | GADD45A, GADD45B, TRAIL, CASP9, CASP6, CIDEC, Smac, c-PARP, c-caspase-3, -7, -8, -9 ↑ MMP, Mcl-1, MMP ↓ | [51] |
| Blister beetles | Cantharidin | U266, RPMI 8226, IM-9 | 5 µM; 24 h | c-caspase -3, -9, c-Bid, Fas ↑ MMP, Bcl-xL ↓ | [52] |
| Dolabella auricularia | Dolastatin | U266, RPMI 8226, IM-9 | 5 nM; 24 h | c-caspase-3, -9, -8, c-Bid, Bax ↑ MMP ↓ | [53] |
| Camellia sinensis (L.) Kuntze | EGCG | OPM1 | 10 µM; 72 h | Fas, Fas ligand, c-caspase -4, p63, DAPK ↑ | [54] |
| Source | Compound | Cell Line | Dose/Duration | Efficacy | References |
|---|---|---|---|---|---|
| Aplidium albicans | Alipidin | MM.1S, MM.1R | MM.1S: 10 nmol/L; MM.1R: 1 nmol/L | G2/M phase arrest | [51] |
| Dolabella auricularia | Dolastatin | RPMI8226 | 0–5 nM; 24 h | G2/M phase arrest | [53] |
| Tripterygium wilfordii Hook. f. | Triptolide | RPMI8226 | 0, 40, 80, 160 nmol/L for 24 h | G2/M phase arrest | [60] |
| Caesalpinia sappan (L.) Tod. | Brazilin | U266 | 60 μM; 6, 12, 24 h | G2/M phase arrest | [37] |
| Boswellia serrata Roxb. ex Colebr. | Boswellic acid | U266, MM.1S | 50 μmol/L;24 h | G2/M phase arrest | [38] |
| Punica granatum L. | Pomegrante extract | U266 | 100, 250 μg/mL; 24 h | G2/M phase, S phase arrest | [46] |
| Source | Compound | Cell Line | Dose/Duration | Mechanism | References |
|---|---|---|---|---|---|
| Brucea javanica (L.) Merr. | Bruceantin (bct) | RPMI 8226 cells, MM-CSC (cancer stem cells) | 0, 25, 50, 100 nM; 24 h | Mechanism N/A | [65] |
| Scutellaria baicalensis Georgi | Wogonin | U266 RPMI 8226 | (in vitro): 20, 40, 80 μM; 24 h (in vivo): 0, 40, 80 mg/kg (i.v. injection); 24 h | <in vitro> VEGF, c-Myc, HIF-1α ↓ <in vivo> c-Myc, HIF-1a, VHL, VEGF ↓ | [66] |
| Citrus paradise Macfad. | Bergamottin | U266 | 100 μM; 0, 6, 12, 24 h | COD-X, VEGF, cyclin D1, IAP-1, Bcl-2, Bcl-xL ↓ | [67] |
| Solenopsis invicta Buren | Solenopsin A | (in vitro) SVR cell proliferation (in vivo) zebrafish model system | (in vitro) 0, 1, 3, 6 µg/mL; 48 h (in vivo) 6 µg/mL; duration N/A | Akt↓, FOXO1a ↓ | [68] |
| Carpobrotus edulis (L.) N.E.Br. | Rutin–Zinc (II) Flavonoid–Metal Complex | RPMI8226 | 17.2–275.6 μM; 24 h | Caspase-3, Caspase-8 ↑ VEGF, cyclin D1 ↓ | [69] |
| Artemisia annua Linn. | Artesunate | RPMI8226 | 3, 6, and 12 μmol/L; 48 h | Mechanism N/A | [70] |
| Source | Compound | Cell Line | Dose/Duration | Mechanism | References |
|---|---|---|---|---|---|
| Coptis chinensis Franch | Berberine | U226, RPMI 8266 | 40, 80, 120, 160 μmol/L 24, 48, 72 h | miR-21, miR-17-92, miR-99a-125b, miR-106-25 ↓ | [35,89,90] |
| Tripterygium wilfordii Hook. F (TWHF) | Triptolide | MM.1S | 2.5–40 ng/mL; 24 h | miR142-5p/miR181a ↓ | [91] |
| Source | Compound | Phase | Patients | Status | Nct Number | References |
|---|---|---|---|---|---|---|
| Agaricus blazei Murrill | Agaricus blazei extract | II | 33 | completed | NCT00970021 | [92,93,94] |
| Curcuma longa Linn | Curcumin (Diferuloylmethane derivative) | pilot study | 33 | completed | NCT00113841 | [40] |
| shark cartilage | Neovastat (AE-941) | II | 125 | completed | NCT00022282 | [95] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, B.; Park, H.; Kim, B. Anticancer Activity and Underlying Mechanism of Phytochemicals against Multiple Myeloma. Int. J. Mol. Sci. 2019, 20, 2302. https://doi.org/10.3390/ijms20092302
Kang B, Park H, Kim B. Anticancer Activity and Underlying Mechanism of Phytochemicals against Multiple Myeloma. International Journal of Molecular Sciences. 2019; 20(9):2302. https://doi.org/10.3390/ijms20092302
Chicago/Turabian StyleKang, Beomku, Hyunmin Park, and Bonglee Kim. 2019. "Anticancer Activity and Underlying Mechanism of Phytochemicals against Multiple Myeloma" International Journal of Molecular Sciences 20, no. 9: 2302. https://doi.org/10.3390/ijms20092302
APA StyleKang, B., Park, H., & Kim, B. (2019). Anticancer Activity and Underlying Mechanism of Phytochemicals against Multiple Myeloma. International Journal of Molecular Sciences, 20(9), 2302. https://doi.org/10.3390/ijms20092302