Immune Functional Analysis of Chitin Deacetylase 3 from the Asian Citrus Psyllid Diaphorina citri
Abstract
:1. Introduction
2. Results
2.1. Bioinformatics Analysis of the DcCDA3 Sequence
2.2. Spatiotemporal Expression Patterns of DcCDA3
2.3. Analysis of the Expression Level of DcCDA3 after 20E Treatment
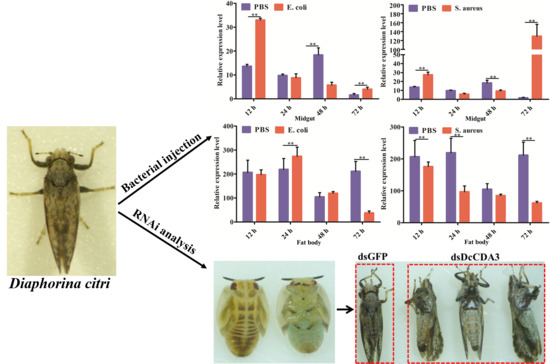
2.4. Functional Analysis of DcCDA3 Based on RNAi
2.5. Expression Profiles of DcCDA3 after Challenge with Bacteria
2.6. Recombinant Protein Expression and Purification
2.7. Enzymatic Assay forRecombinant DcCDA3 Activities
2.8. Antibacterial Activity of Recombinant DcCDA3
3. Discussion
4. Materials and Methods
4.1. Diaphorina Citri Rearing and Collection
4.2. Bacterial Challenge and Sample Preparation
4.3. Preparation and Treatment of 20E
4.4. RNA Isolation and cDNA Synthesis
4.5. Identification of DcCDA3 and Bioinformatics Analysis
4.6. Reverse Transcription Quantitative PCR (RT-qPCR) Analysis of DcCDA3 Expression Levels
4.7. dsRNA Synthesis and DcCDA3 RNAi Analysis
4.8. Prokaryotic Expression and Protein Purification
4.9. Enzymatic Assay for Recombinant DcCDA3 Activity
4.10. Antibacterial Assay for Recombinant DcCDA3 Protein
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CDA | Chitin deacetylase |
| ACP | Asian citrus psyllid |
| HLB | Huanglongbing |
| RNAi | RNA interference |
| 20E | 20-Hydroxyecdysone |
| CBD | Chitin-binding peritrophin-A domain |
| LDLa | Low-density lipoprotein receptor class A |
| CHS | Chitin synthase |
| CHT | Chitinase |
References
- Boina, D.R.; Salyani, M.; Stelinski, L.L. Chemical control of the Asian citrus psyllid, Diaphorina citri Kuwayama. Proc. Fla. State Hortic. Soc. 2009, 122, 176–180. [Google Scholar]
- Grafton-Cardwell, E.E.; Stelinski, L.L.; Stansly, P.A. Biology and management of Asian citrus psyllid, vector of the huanglongbing pathogens. Annu. Rev. Entomol. 2013, 58, 413–432. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.K.; Hu, R.F.; Pray, C.; Qiao, F.B.; Rozelle, S. Biotechnology as an alternative to chemical pesticides: A case study of Bt cotton in China. Agric. Econ. 2015, 29, 55–67. [Google Scholar] [CrossRef]
- Boina, D.R.; Onagbola, E.O.; Salyani, M.; Stelinski, L.L. Antifeedant and sublethal effects of imidacloprid on Asian citrus psyllid, Diaphorina citri. Pest Manag. Sci. 2010, 65, 870–877. [Google Scholar] [CrossRef]
- Zhao, Y.J.; Sui, X.Y.; Xu, L.J.; Liu, G.Y.; Lu, L.H.; You, M.S.; Xie, C.J.; Li, B.Y.; Ni, Z.F.; Liang, R.Q. Plant-mediated RNAi of grain aphid CHS1 gene confers common wheat resistance against aphids. Pest Manag. Sci. 2018, 74, 2754–2760. [Google Scholar] [CrossRef]
- Shang, F.; Xiong, Y.; Xia, W.K.; Wei, D.D.; Wei, D.; Wang, J.J. Identification, characterization and functional analysis of a chitin synthase gene in the brown citrus aphid, Toxoptera citricida (Hemiptera, Aphididae). Insect Mol. Biol. 2016, 25, 422–430. [Google Scholar] [CrossRef]
- Yu, X.D.; Gowda, S.; Killiny, N. Double-stranded RNA delivery through soaking mediates silencing of the muscle protein 20 and increases mortality to the Asian citrus psyllid, Diaphorina citri. Pest Manag. Sci. 2017, 73, 1846–1853. [Google Scholar] [CrossRef]
- Killiny, N.; Hajeri, S.; Tiwari, S.; Gowda, S.; Stelinski, L.L. Double-stranded RNA uptake through topical application, mediates silencing of five CYP4 genes and suppresses insecticide resistance in Diaphorina citri. PLoS ONE 2014, 9, e110536. [Google Scholar] [CrossRef] [Green Version]
- Merzendorfer, H. Insect chitin synthases: A review. J. Comp. Physiol. B 2006, 176, 1–15. [Google Scholar] [CrossRef]
- Rebers, J.E.; Willis, J.H. A conserved domain in arthropod cuticular proteins binds chitin. Insect Biochem. Mol. Biol. 2001, 31, 1083–1093. [Google Scholar] [CrossRef]
- Tsigos, I.; Martinou, A.; Kafetzopoulos, D.; Bouriotis, V. Chitin deacetylases: New, versatile tools in biotechnology. Trends Biotechnol. 2000, 18, 305–312. [Google Scholar] [CrossRef]
- Guo, W.; Li, G.X.; Pang, Y.; Wang, P. A novel chitin-binding protein identified from the peritrophic membrane of the cabbage looper, Trichoplusia ni. Insect Biochem. Mol. Biol. 2005, 35, 1224–1234. [Google Scholar] [CrossRef]
- Liu, S.H.; Li, H.F.; Yang, Y.; Yang, R.L.; Yang, W.J.; Jiang, H.B.; Dou, W.; Smagghe, G.; Wang, J.J. Genome-wide identification of chitinase and chitin deacetylase gene families in the oriental fruit fly, Bactrocera dorsalis (Hendel). Comp. Biochem. Phys. D 2018, 27, 13–22. [Google Scholar] [CrossRef]
- Zhang, M.; Ji, Y.N.; Zhang, X.B.; Ma, P.J.; Wang, Y.W.; Moussian, B.; Zhang, J.Z. The putative chitin deacetylases Serpentine and Vermiform have non-redundant functions during Drosophila wing development. Insect Biochem. Mol. Biol. 2019, 110, 128–135. [Google Scholar] [CrossRef]
- Yu, R.R.; Ding, G.W.; Guo, Y.P.; Ma, E.B.; Zhang, J.Z. Molecular characterization and biological function of chitin deacetylase 2 genes in Locusta migratoria. Sci. Agric. Sin. 2014, 47, 1321–1329. [Google Scholar]
- Yu, H.Z.; Liu, M.H.; Wang, X.Y.; Yang, X.; Wang, W.L.; Geng, L.; Yu, D.; Liu, X.L.; Liu, G.Y.; Xu, J.P. Identification and expression profiles of chitin deacetylase genes in the rice leaf folder, Cnaphalocrocis medinalis. J. Asia Pac. Entomol. 2016, 19, 691–696. [Google Scholar] [CrossRef]
- Xi, Y.; Pan, P.L.; Ye, Y.X.; Yu, B.; Zhang, C.X. Chitin deacetylase family genes in the brown planthopper, Nilaparvata lugens (Hemiptera: Delphacidae). Insect Mol. Biol. 2015, 23, 695–705. [Google Scholar] [CrossRef]
- Dixit, R.; Arakane, Y.; Specht, C.A.; Richard, C.; Kramer, K.J.; Beeman, R.W.; Muthukrishnan, S. Domain organization and phylogenetic analysis of proteins from the chitin deacetylase gene family of Tribolium castaneum and three other species of insects. Insect Biochem. Mol. Biol. 2008, 38, 440–451. [Google Scholar] [CrossRef] [Green Version]
- Chaudhari, S.S.; Arakane, Y.; Specht, C.A.; Moussian, B.; Boyle, D.L.; Park, Y.; Kramer, K.J.; Beeman, R.W.; Muthukrishnan, S. Knickkopf protein protects and organizes chitin in the newly synthesized insect exoskeleton. Proc. Natl. Acad. Sci. USA 2011, 108, 17028–17033. [Google Scholar] [CrossRef] [Green Version]
- Yan, X.P.; Zhao, D.; Zhang, Y.K.; Guo, W.; Wang, W.; Zhao, K.L.; Gao, Y.J.; Wang, X.Y. Identification and characterization of chitin deacetylase2 from the American white moth, Hyphantria cunea (Drury). Gene 2018, 670, 98–105. [Google Scholar] [CrossRef]
- Tetreau, G.; Cao, X.L.; Chen, Y.R.; Muthukrishnan, S.; Jiang, H.B.; Blissard, G.W.; Kanost, M.R.; Wang, P. Overview of chitin metabolism enzymes in Manduca sexta: Identification, domain organization, phylogenetic analysis and gene expression. Insect Biochem. Mol. Biol. 2015, 62, 114–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arakane, Y.; Dixit, R.; Begum, K.; Park, Y.; Specht, C.A.; Merzendorfer, H.; Kramer, K.J.; Muthukrishnan, S.; Beeman, R.W. Analysis of functions of the chitin deacetylase gene family in Tribolium castaneum. Insect Biochem. Mol. Biol. 2009, 39, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Rudall, K.M. The Chitin/Protein Complexes of Insect Cuticles. Adv. Insect Physiol. 1963, 1, 257–313. [Google Scholar]
- Togawa, T.; Nakato, H.; Izumi, S. Analysis of the chitin recognition mechanism of cuticle proteins from the soft cuticle of the silkworm, Bombyx mori. Insect Biochem. Mol. Biol. 2004, 34, 1059–1067. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Y.; Yan, J.M.; Liu, Q.; Zhang, Y.H.; Gong, J.; Hou, Y. Genome-wide analysis and hormone regulation of chitin deacetylases in silkworm. Int. J. Mol. Sci. 2019, 20, 1679. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.J.; Xu, K.K.; Yan, X.; Chen, C.X.; Cao, Y.; Meng, Y.L.; Li, C. Functional characterization of chitin deacetylase 1 gene disrupting larval–pupal transition in the drugstore beetle using RNA interference. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2018, 219–220, 10–16. [Google Scholar] [CrossRef]
- Arp, A.P.; Martini, X.; Pelz-Stelinski, K.S. Innate immune system capabilities of the Asian citrus psyllid, Diaphorina citri. J. Invertebr. Pathol. 2017, 148, 94–101. [Google Scholar] [CrossRef]
- Zhao, Y.; Park, R.D.; Muzzarelli, R.A. Chitin deacetylases: Properties and applications. Mar. Drugs. 2010, 8, 24–46. [Google Scholar] [CrossRef]
- Sun, Y.Y.; Zhang, J.Q.; Xiang, J.H. Immune function against bacteria of chitin deacetylase 1 (EcCDA1) from Exopalaemon carinicauda. Fish Shellfish Immunol. 2018, 75, 115–123. [Google Scholar] [CrossRef]
- Cuesta, A.; Esteban, M.A.; Mesequer, J. In vitro effect of chitin particles on the innate cellular immune system of gilthead seabream (Sparus aurata L.). Fish Shellfish Immunol. 2003, 15, 1–11. [Google Scholar] [CrossRef]
- Yu, H.Z.; Huang, Y.L.; Li, N.Y.; Xie, Y.X.; Zhou, C.H.; Lu, Z.J. Potential roles of two cathepsin genes, DcCath-L and DcCath-O in the innate immune response of Diaphorina citri. J. Asia Pac. Entomol. 2019, 22, 1060–1069. [Google Scholar] [CrossRef]
- Gu, J.H.; Shao, Y.; Zhang, C.W.; Liu, Z.W.; Zhang, Y.J. Characterization of putative soluble and membrane-bound trehalases in a hemipteran insect, Nilaparvata lugens. J. Insect Physiol. 2009, 55, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.W.; Wang, X.H.; Tan, X.; Xia, Q.Y.; Xiang, Z.H.; Zhao, P. Identification and molecular characterization of a chitin deacetylase from Bombyx mori peritrophic membrane. Int. J. Mol. Sci. 2014, 15, 1946–1961. [Google Scholar] [CrossRef] [PubMed] [Green Version]









| Primers | Sequences | Purpose |
|---|---|---|
| DcCDA3-RT-F | CAGATGAGGTCTTGGAGTGGCT | RT-qPCR |
| DcCDA3-RT-R | GTTCTTTGCTTGACTTTGGGTTG | |
| GAPDH-F | CATGGCAAGTTCAACGGTGA | |
| GAPDH-R | CGATGCCTTCTCAATGGTGG | |
| ds-DcCDA3-F | TAATACGACTCACTATAGGGAGAAGGATAAGAGCAAGGAC | dsRNA synthesis |
| ds-DcCDA3-R | TAATACGACTCACTATAGGGTACTCGTGGGAGATGAAGAA | |
| ds-GFP-F | GGATCCTAATACGACTCACTATAGGCAGTGCTTCAGCCGCTACCC | |
| ds-GFP-R | GGATCCTAATACGACTCACTATAGGACTCCAGCAGGACCATGTGAT | |
| DcCDA3-Ex-F | CGGGATCCCAGAAGGATAAGAGCAA | Protein expression |
| DcCDA3-Ex-R | CGCTCGAGCCTGGTAGCAGAGATGC |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, H.-Z.; Li, N.-Y.; Li, B.; Toufeeq, S.; Xie, Y.-X.; Huang, Y.-L.; Du, Y.-M.; Zeng, X.-D.; Zhu, B.; Lu, Z.-J. Immune Functional Analysis of Chitin Deacetylase 3 from the Asian Citrus Psyllid Diaphorina citri. Int. J. Mol. Sci. 2020, 21, 64. https://doi.org/10.3390/ijms21010064
Yu H-Z, Li N-Y, Li B, Toufeeq S, Xie Y-X, Huang Y-L, Du Y-M, Zeng X-D, Zhu B, Lu Z-J. Immune Functional Analysis of Chitin Deacetylase 3 from the Asian Citrus Psyllid Diaphorina citri. International Journal of Molecular Sciences. 2020; 21(1):64. https://doi.org/10.3390/ijms21010064
Chicago/Turabian StyleYu, Hai-Zhong, Ning-Yan Li, Bing Li, Shahzad Toufeeq, Yan-Xin Xie, Yu-Ling Huang, Yi-Ming Du, Xiang-Dong Zeng, Bo Zhu, and Zhan-Jun Lu. 2020. "Immune Functional Analysis of Chitin Deacetylase 3 from the Asian Citrus Psyllid Diaphorina citri" International Journal of Molecular Sciences 21, no. 1: 64. https://doi.org/10.3390/ijms21010064
APA StyleYu, H. -Z., Li, N. -Y., Li, B., Toufeeq, S., Xie, Y. -X., Huang, Y. -L., Du, Y. -M., Zeng, X. -D., Zhu, B., & Lu, Z. -J. (2020). Immune Functional Analysis of Chitin Deacetylase 3 from the Asian Citrus Psyllid Diaphorina citri. International Journal of Molecular Sciences, 21(1), 64. https://doi.org/10.3390/ijms21010064