Effects of Regorafenib, a Multi-Kinase Inhibitor, on Conjunctival Scarring in a Canine Filtration Surgery Model in Comparison with Mitomycin-C
Abstract
:1. Introduction
2. Results
2.1. Experiment 1 (Ex 1): Comparison of the Effects in the Regorafenib and Control Groups
2.1.1. IOP Change
2.1.2. Bleb Score
2.1.3. Subconjunctival/Scleral Area Ratio
2.1.4. Density of Collagen in Subconjunctival Tissue
2.1.5. Density of Vessels in Subconjunctival Tissue
2.1.6. Vimentin-, TGF-β-, Proliferative Cell Nuclear Antigen (PCNA)-, and αSMA-Positive Cells
2.2. Experiment 2 (Ex 2): Comparison of the Effects in the Regorafenib and MMC Groups
2.2.1. IOP Change
2.2.2. Bleb Score
2.2.3. Ultrasonic Biomicroscope (UBM) Assessments
2.2.4. Density of Collagen in Subconjunctival Tissue
2.2.5. Density of Vessels in the Subconjunctival Tissue
2.2.6. Vimentin-, TGF-β-, PCNA-, and SMA-Positive Cells
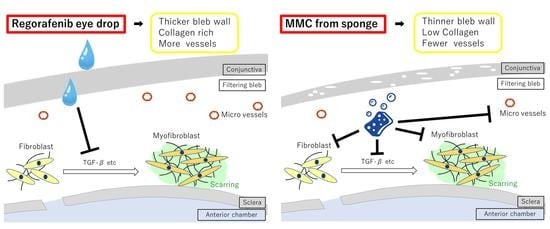
3. Discussion
4. Materials and Methods
4.1. Drugs
4.2. Animals and IOP Measurements
4.3. Glaucoma Filtration Surgery Model
4.4. Experiment Protocol
4.4.1. Experiment 1: Comparison of the Effects in the Regorafenib and Control Groups
4.4.2. Experiment 2: Comparison of the Effects in the Regorafenib and MMC Groups
4.5. Bleb Scores
4.6. Ultrasound Biomicroscopy (UBM)
4.7. Histological Examination
4.8. Masking
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Quigley, H.A.; Broman, A.T. The number of people with glaucoma worldwide in 2010 and 2020. Br. J. Ophthalmol. 2006, 90, 262–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heijl, A.; Leske, M.C.; Bengtsson, B.; Hyman, L.; Bengtsson, B.; Hussein, M. Early Manifest Glaucoma Trial Group. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002, 120, 1268–1279. [Google Scholar] [CrossRef] [PubMed]
- Hitchings, R. Initial treatment for open-angle glaucoma- medical, laser, or surgical? Surgery is the treatment of choice for open-angle glaucoma. Arch. Ophthalmol. 1998, 116, 241–242. [Google Scholar] [CrossRef] [PubMed]
- Skuta, G.L.; Parrish, R.K. Wound healing in glaucoma filtering surgery. Surv. Ophthalmol. 1987, 32, 149–170. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, R.C.; Li, J.; Chalam, K.V.; Tripathi, B.J. Expression of growth factor mRNAs by human Tenon’s capsule fibroblasts. Exp. Eye Res. 1996, 63, 339–346. [Google Scholar] [CrossRef]
- Chen, C. Enhanced intraocular pressure controlling effectiveness of trabeculectomy by local application of mitomycin C. Trans. Asia Pac. Acad. Ophthalmol. 1983, 9, 172–177. [Google Scholar]
- Cheung, J.C.; Wright, M.M.; Murali, S.; Pederson, J.E. Intermediate-term outcome of variable dose mitomycin C filtering surgery. Ophthalmology 1997, 104, 143–149. [Google Scholar] [CrossRef]
- Perkins, T.W.; Gangnon, R.; Ladd, W.; Kaufman, P.L.; Heatley, G.A. Trabeculectomy with mitomycin C: intermediate-term results. J. Glaucoma. 1998, 7, 230–236. [Google Scholar] [CrossRef]
- Wilkins, M.; Indar, A.; Wormald, R. Intra-operative mitomycin C for glaucoma surgery. Cochrane Database Syst Rev. 2005, 19, CD002897. [Google Scholar]
- Mochizuki, K.; Jikihara, S.; Ando, Y.; Hori, N.; Yamamoto, T.; Kitazawa, Y. Incidence of delayed onset infection after trabeculectomy with adjunctive mitomycin C or 5-fluorouracil treatment. Br. J. Ophthalmol. 1997, 81, 877–883. [Google Scholar] [CrossRef] [Green Version]
- Greenfield, D.S.; Suner, I.J.; Miller, M.P.; Kangas, T.A.; Palmberg, P.F.; Flynn, H.W., Jr. Endophthalmitis after filtering surgery with mitomycin. Arch. Ophthalmol. 1996, 114, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Sawada, A.; Mayama, C.; Araie, M.; Ohkubo, S.; Sugiyama, K.; Kuwayama, Y. Collaborative Bleb-Related Infection Incidence and Treatment Study Group. The 5-year incidence of bleb-related infection and its risk factors after filtering surgeries with adjunctive mitomycin C: collaborative bleb-related infection incidence and treatment study 2. Ophthalmology. 2014, 121, 1001–1006. [Google Scholar] [PubMed]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Wipff, P.J.; Rifkin, D.B.; Meister, J.J.; Hinz, B. Myofibroblast contraction activates latent TGF-beta1 from the extracellular matrix. J. Cell Biol. 2007, 179, 1311–1323. [Google Scholar] [CrossRef] [Green Version]
- Park, H.Y.; Kim, J.H.; Park, C.K. VEGF induces TGF-β1 expression and myofibroblast transformation after glaucoma surgery. Am. J Pathol. 2013, 182, 2147–2154. [Google Scholar] [CrossRef]
- Gabbiani, G. The myofibroblast in wound healing and fibrocontractive diseases. J. Pathol. 2003, 200, 500–503. [Google Scholar] [CrossRef]
- Desmouliere, A.; Redard, M.; Darby, I.; Gabbiani, G. Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am. J. Pathol. 1995, 146, 56–66. [Google Scholar]
- CAT-152 0102 Trabeculectomy Study Group; Khaw, P.; Grehn, F.; Hollo, G.; Overton, B.; Wilson, R.; Vogel, R.; Smith, Z. A phase III study of subconjunctival human anti-transforming growth factor β2 monoclonal antibody (CAT-152) to prevent scarring after first-time trabeculectomy. Ophthalmology 2007, 114, 1822–1830. [Google Scholar]
- Pro, M.J.; Freidl, K.B.; Neylan, C.J.; Sawchyn, A.K.; Wizov, S.S.; Moster, M.R. Ranibizumab versus mitomycin C in primary trabeculectomy—A pilot study. Curr. Eye Res. 2015, 40, 510–515. [Google Scholar] [CrossRef]
- Freiberg, F.J.; Matlach, J.; Grehn, F.; Karl, S.; Klink, T. Postoperative subconjunctival bevacizumab injection as an adjunct to 5-fluorouracil in the management of scarring after trabeculectomy. Clin. Ophthalmol. 2013, 7, 1211–1217. [Google Scholar] [CrossRef] [Green Version]
- Xiong, Q.; Li, Z.; Li, Z.; Zhu, Y.; Abdulhalim, S.; Wang, P.; Cai, X. Anti-VEGF agents with or without antimetabolites in trabeculectomy for glaucoma: A meta-analysis. PLoS ONE 2014, 9, e88403. [Google Scholar] [CrossRef] [PubMed]
- Denk, P.O.; Hoppe, J.; Hoppe, V.; Knorr, M. Effect of growth factors on the activation of human Tenon’s capsule fibroblasts. Curr Eye Res. 2003, 27, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Knorr, M.; Volker, M.; Denk, P.O.; Wunderlich, K.; Thiel, H.J. Proliferative response of cultured human Tenon’s capsule fibroblasts to platelet-derived growth factor isoforms. Graefes Arch Clin. Exp. Ophthalmol. 1997, 235, 667–671. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, S.M.; Dumas, J.; Adnane, L.; Lynch, M.; Carter, C.A.; Schütz, G.; Thierauch, K.H.; Zopf, D. Regorafenib (BAY 73-4506): A new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int. J. Cancer. 2011, 129, 245–255. [Google Scholar] [CrossRef]
- Ettrich, T.J.; Seufferlein, T. Regorafenib. Recent Results Cancer Res. 2018, 211, 45–56. [Google Scholar]
- Kenan, E.; Turgut, B.; Akin, M.M.; Demir, T. The suppression of wound healing response with sirolimus and sunitinib following experimental trabeculectomy in a rabbit model. Curr. Eye Res. 2016, 41, 367–376. [Google Scholar]
- Zimmermann, T.; Höchel, J.; Becka, M.; Boettger, M.K.; Rohde, B.; Schug, B.; Kunert, K.S.; Donath, F. Topical administration of regorafenib eye drop: phase 1 dose-escalation study in healthy volunteers. Br. J. Clin. Pharmacol. 2018, 84, 865–875. [Google Scholar] [CrossRef] [Green Version]
- Joussen, A.M.; Wolf, S.; Kaiser, P.K.; Boyer, D.; Schmelter, T.; Sandbrink, R.; Zeitz, O.; Deeg, G.; Richter, A.; Zimmermann, T.; et al. The Developing Regorafenib Eye drops for neovascular Age-related Macular degeneration (DREAM) study: An open-label phase II trial. Br. J. Clin. Pharmacol. 2019, 85, 347–355. [Google Scholar] [CrossRef] [Green Version]
- Samuelson, D.A.; Gelatt, K.N. Aqueous outflow in the beagle. II. Postnatal morphologic development of the iridocorneal angle: corneoscleral trabecular meshwork and angular aqueous plexus. Curr. Eye Res. 1984, 3, 795–807. [Google Scholar] [CrossRef]
- Kojima, S.; Sugiyama, T.; Takai, S.; Jin, D.; Shibata, M.; Oku, H.; Tabata, Y.; Ikeda, T. Effects of gelatin hydrogel containing chymase inhibitor on scarring in a canine filtration surgery model. Invest Ophthalmol Vis Sci. 2011, 52, 7672–7680. [Google Scholar] [CrossRef]
- Maeda, M.; Kojima, S.; Sugiyama, T.; Jin, D.; Takai, S.; Oku, H.; Kohmoto, R.; Ueki, M.; Ikeda, T. Effects of gelatin hydrogel containing anti-transforming growth factor-β antibody in a canine filtration surgery model. Int. J. Mol. Sci. 2017, 18, 985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Salhy, A.A.; Elseht, R.M.; Al Maria, A.F.; Shalaby, S.A.M.E.; Hossein, T.R. Functional evaluation of the filtering bleb by ultrasound biomicroscopy after trabeculectomy with mitomycin C. Int. J. Ophthalmol. 2018, 11, 245–250. [Google Scholar] [PubMed]
- Yamamoto, T.; Sakuma, T.; Kitazawa, Y. An ultrasound biomicroscopic study of filtering blebs after mitomycin C trabeculectomy. Ophthalmology 1995, 102, 1770–1776. [Google Scholar] [CrossRef]
- Yamamoto, T.; Varani, J.; Soong, H.K.; Lichter, P.R. Effects of 5-fluorouracil and mitomycin C on cultured rabbit subconjunctival fibroblasts. Ophthalmology 1990, 97, 1204–1210. [Google Scholar] [CrossRef]
- Gross, R.L. Collagen type I and III synthesis by Tenon’s capsule fibroblasts in culture: individual patient characteristics and response to mitomycin C, 5-fluorouracil, and ascorbic acid. Trans Am. Ophthalmol. Soc. 1999, 97, 513–543. [Google Scholar]
- Seong, G.J.; Park, C.; Kim, C.Y.; Hong, Y.J.; So, H.S.; Kim, S.D.; Park, R. Mitomycin-C induces the apoptosis of human Tenon’s capsule fibroblast by activation of c-Jun N-terminal kinase 1 and caspase-3 protease. Invest. Ophthalmol. Vis. Sci. 2005, 46, 3545–3552. [Google Scholar] [CrossRef] [Green Version]
- Horita, S.; Watanabe, M.; Katagiri, M.; Nakamura, H.; Haniuda, H.; Nakazato, T.; Kagawa, Y. Species differences in ocular pharmacokinetics and pharmacological activities of regorafenib and pazopanib eye-drops among rats, rabbits and monkeys. Pharmacol. Res. Perspect. 2019, 20, e00545. [Google Scholar] [CrossRef]
- Perkins, T.W.; Faha, B.; Ni, M.; Kiland, J.A.; Poulsen, G.L.; Antelman, D.; Atencio, I.; Shinoda, J.; Sinha, D.; Brumback, L.; et al. Adenovirus-mediated gene therapy using human p21WAF-1/Cip-1 to prevent wound healing in a rabbit model of glaucoma filtration surgery. Arch. Ophthalmol. 2002, 120, 941–949. [Google Scholar] [CrossRef] [Green Version]









| Index | Control Group | Regorafenib Group | p-Value (Mann–Whitney U) |
|---|---|---|---|
| Ratio of the conjunctival area to the scleral area | 1.1 ± 0.1 | 0.8 ± 0.2 | 0.025 |
| Collagen density of subconjunctival tissue, % | 89.3 ± 5.9 | 75.5 ± 9.1 | 0.025 |
| Density of vessels in subconjunctival tissue, per·mm2 | 14.1 ± 4.7 | 8.3 ± 4.0 | 0.03 |
| Density of vimentin-positive cells, per·mm2 | 33.8 ± 15.4 | 10.3 ± 9.3 | 0.02 |
| Density of TGFβ-positive cells, per·mm2 | 18.3 ± 5.7 | 5.6 ± 3.9 | 0.007 |
| Density of PCNA-positive cells, per·mm2 | 13.8 ± 6.8 | 6.3 ± 3.0 | 0.045 |
| Density of αSMA-positive cells, per·mm2 | 3.3 ± 1.3 | 0.3 ± 0.5 | 0.004 |
| Index | Regorafenib Group | MMC Group | p-Value (Mann–Whitney U) |
|---|---|---|---|
| Ratio of the conjunctival area to the scleral area | 1.5 ± 0.4 | 1.4 ± 0.3 | 0.45 |
| Collagen density of subconjunctival tissue, % | 82.5 ± 8.1 | 62.3 ± 17.6 | 0.01 |
| Density of vessels in the subconjunctival tissue, per·mm2 | 11.5 ± 2.0 | 6.5 ± 1.9 | 0.01 |
| Density of vimentin-positive cells, per·mm2 | 5.5 ± 0.7 | 5.0 ± 0.2 | 0.63 |
| Density of TGFβ-positive cells, per·mm2 | 3.8 ± 0.9 | 3.5 ± 1.2 | 0.63 |
| Density of PCNA-positive cells, per·mm2 | 6.3 ± 1.3 | 5.3 ± 1.2 | 0.2 |
| Density of αSMA-positive cells, per·mm2 | 1.1 ± 0.9 | 1.0 ± 0.8 | 0.87 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nemoto, E.; Kojima, S.; Sugiyama, T.; Jin, D.; Takai, S.; Maeda, M.; Kohmoto, R.; Ueki, M.; Oku, H.; Ikeda, T. Effects of Regorafenib, a Multi-Kinase Inhibitor, on Conjunctival Scarring in a Canine Filtration Surgery Model in Comparison with Mitomycin-C. Int. J. Mol. Sci. 2020, 21, 63. https://doi.org/10.3390/ijms21010063
Nemoto E, Kojima S, Sugiyama T, Jin D, Takai S, Maeda M, Kohmoto R, Ueki M, Oku H, Ikeda T. Effects of Regorafenib, a Multi-Kinase Inhibitor, on Conjunctival Scarring in a Canine Filtration Surgery Model in Comparison with Mitomycin-C. International Journal of Molecular Sciences. 2020; 21(1):63. https://doi.org/10.3390/ijms21010063
Chicago/Turabian StyleNemoto, Emika, Shota Kojima, Tetsuya Sugiyama, Denan Jin, Shinji Takai, Michiko Maeda, Ryohsuke Kohmoto, Mari Ueki, Hidehiro Oku, and Tsunehiko Ikeda. 2020. "Effects of Regorafenib, a Multi-Kinase Inhibitor, on Conjunctival Scarring in a Canine Filtration Surgery Model in Comparison with Mitomycin-C" International Journal of Molecular Sciences 21, no. 1: 63. https://doi.org/10.3390/ijms21010063
APA StyleNemoto, E., Kojima, S., Sugiyama, T., Jin, D., Takai, S., Maeda, M., Kohmoto, R., Ueki, M., Oku, H., & Ikeda, T. (2020). Effects of Regorafenib, a Multi-Kinase Inhibitor, on Conjunctival Scarring in a Canine Filtration Surgery Model in Comparison with Mitomycin-C. International Journal of Molecular Sciences, 21(1), 63. https://doi.org/10.3390/ijms21010063