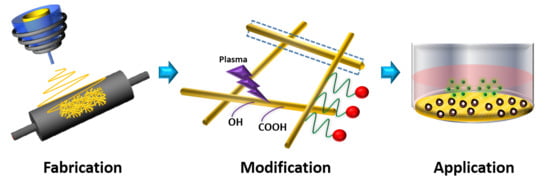
Recent Developments in Nanofiber Fabrication and Modification for Bone Tissue Engineering
Abstract
:1. Introduction
2. Nanofibrous Scaffold Fabrication
2.1. Conventional Electrospinning
2.2. Multi-Axial Electrospinning (Core–Shell Nanofiber)
2.3. Electrospinning with a Modified Collector (Oriented/Aligned Nanofiber)
2.4. Melt-Electrospinning (3-Dimensional Fiber)
3. Modification/Post-Processing of Nanofiber for Bone Tissue Engineering
3.1. Surface Modification: Plasma and Laser Treatment
3.2. Surface Functionalization
3.3. Inorganic Combination or Hydroxyapatite Deposition (Reinforced) Mineralization/Inorganic Hybrid
3.4. Crosslinking Methods
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, X.; Ma, P.X. Polymeric scaffolds for bone tissue engineering. Ann. Biomed. Eng. 2004, 32, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Leong, K.-F.; Du, Z.; Chua, C.-K. The design of scaffolds for use in tissue engineering. Part, I. Traditional factors. Tissue Eng. 2001, 7, 679–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshimoto, H.; Shin, Y.; Terai, H.; Vacanti, J. A biodegradable nanofiber scaffold by electrospinning and its potential for bone tissue engineering. Biomaterials 2003, 24, 2077–2082. [Google Scholar] [CrossRef]
- Hutmacher, D.W. Scaffolds in tissue engineering bone and cartilage. Biomaterials 2000, 21, 2529–2543. [Google Scholar] [CrossRef]
- Balagangadharan, K.; Dhivya, S.; Selvamurugan, N. Chitosan based nanofibers in bone tissue engineering. Int. J. Biol. Macromol. 2017, 104, 1372–1382. [Google Scholar] [CrossRef] [PubMed]
- Khajavi, R.; Abbasipour, M.; Bahador, A. Electrospun biodegradable nanofibers scaffolds for bone tissue engineering. J. Appl. Polym. Sci. 2016, 133, 42883. [Google Scholar] [CrossRef]
- Ingavle, G.C.; Leach, J.K. Advancements in electrospinning of polymeric nanofibrous scaffolds for tissue engineering. Tissue Eng. Part B Rev. 2013, 20, 277–293. [Google Scholar] [CrossRef]
- Rezvani, Z.; Venugopal, J.R.; Urbanska, A.M.; Mills, D.K.; Ramakrishna, S.; Mozafari, M. A bird’s eye view on the use of electrospun nanofibrous scaffolds for bone tissue engineering: Current state-of-the-art, emerging directions and future trends. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 2181–2200. [Google Scholar] [CrossRef]
- Agarwal, S.; Greiner, A.; Wendorff, J.H. Functional materials by electrospinning of polymers. Prog. Polym. Sci. 2013, 38, 963–991. [Google Scholar] [CrossRef]
- Liu, H.; Ding, X.; Zhou, G.; Li, P.; Wei, X.; Fan, Y. Electrospinning of nanofibers for tissue engineering applications. J. Nanomater. 2013, 2013, 3. [Google Scholar] [CrossRef] [Green Version]
- Zhou, C.; Shi, Q.; Guo, W.; Terrell, L.; Qureshi, A.T.; Hayes, D.J.; Wu, Q. Electrospun bio-nanocomposite scaffolds for bone tissue engineering by cellulose nanocrystals reinforcing maleic anhydride grafted PLA. ACS Appl. Mater. Interfaces 2013, 5, 3847–3854. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.S.; Kim, T.G.; Park, T.G. Surface-functionalized electrospun nanofibers for tissue engineering and drug delivery. Adv. Drug Deliv. Rev. 2009, 61, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Ramos, D.; Lee, P.; Liang, D.; Yu, X.; Kumbar, S.G. Collagen functionalized bioactive nanofiber matrices for osteogenic differentiation of mesenchymal stem cells: Bone tissue engineering. J. Biomed. Nanotechnol. 2014, 10, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Song, J.; Ji, P.; Zhang, X.; Li, X.; Xu, X.; Wang, M.; Zhang, S.; Deng, Y.; Deng, F. Polydopamine-templated hydroxyapatite reinforced polycaprolactone composite nanofibers with enhanced cytocompatibility and osteogenesis for bone tissue engineering. ACS Appl. Mater. Interfaces 2016, 8, 3499–3515. [Google Scholar] [CrossRef] [PubMed]
- Stegen, S.; van Gastel, N.; Carmeliet, G. Bringing new life to damaged bone: The importance of angiogenesis in bone repair and regeneration. Bone 2015, 70, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Taipale, J.; Keski-Oja, J. Growth factors in the extracellular matrix. Faseb J. 1997, 11, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Solheim, E. Growth factors in bone. Int. Orthop. 1998, 22, 410–416. [Google Scholar] [CrossRef] [Green Version]
- Gittens, S.A.; Uludag, H. Growth factor delivery for bone tissue engineering. J. Drug Target. 2001, 9, 407–429. [Google Scholar] [CrossRef]
- Tayalia, P.; Mooney, D.J. Controlled growth factor delivery for tissue engineering. Adv. Mater. 2009, 21, 3269–3285. [Google Scholar] [CrossRef]
- Schultz, G.S.; Wysocki, A. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen 2009, 17, 153–162. [Google Scholar] [CrossRef]
- Vo, T.N.; Kasper, F.K.; Mikos, A.G. Strategies for controlled delivery of growth factors and cells for bone regeneration. Adv. Drug Deliv. Rev. 2012, 64, 1292–1309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reichert, J.C.; Cipitria, A.; Epari, D.R.; Saifzadeh, S.; Krishnakanth, P.; Berner, A.; Woodruff, M.A.; Schell, H.; Mehta, M.; Schuetz, M.A.; et al. A Tissue Engineering Solution for Segmental Defect Regeneration in Load-Bearing Long Bones. Sci. Transl. Med. 2012, 4, 141ra193. [Google Scholar] [CrossRef] [PubMed]
- Kowalczewski, C.J.; Saul, J.M. Biomaterials for the delivery of growth factors and other therapeutic agents in tissue engineering approaches to bone regeneration. Front. Pharmacol. 2018, 9, 513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charoenlarp, P.; Rajendran, A.K.; Iseki, S. Role of fibroblast growth factors in bone regeneration. Inflamm. Regen. 2017, 37, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rose, F.R.; Hou, Q.; Oreffo, R.O. Delivery systems for bone growth factors - the new players in skeletal regeneration. J. Pharm. Pharm. 2004, 56, 415–427. [Google Scholar] [CrossRef]
- Sankar, S.; Sharma, C.S.; Rath, S.N.; Ramakrishna, S. Electrospun nanofibres to mimic natural hierarchical structure of tissues: Application in musculoskeletal regeneration. J. Tissue Eng. Regen. Med. 2018, 12, e604–e619. [Google Scholar] [CrossRef]
- Sui, G.; Yang, X.; Mei, F.; Hu, X.; Chen, G.; Deng, X.; Ryu, S. Poly-L-lactic acid/hydroxyapatite hybrid membrane for bone tissue regeneration. J. Biomed. Mater. Res. Part A 2007, 82A, 445–454. [Google Scholar] [CrossRef]
- Cui, W.; Li, X.; Zhou, S.; Weng, J. In situ growth of hydroxyapatite within electrospun poly (DL-lactide) fibers. J. Biomed. Mater. Res. Part A 2007, 82A, 831–841. [Google Scholar] [CrossRef]
- Sahoo, S.; Ang, L.T.; Goh, J.C.-H.; Toh, S.-L. Growth factor delivery through electrospun nanofibers in scaffolds for tissue engineering applications. J. Biomed. Mater. Res. Part A 2010, 93A, 1539–1550. [Google Scholar] [CrossRef]
- Liu, W.; Thomopoulos, S.; Xia, Y. Electrospun Nanofibers for Regenerative Medicine. Adv. Healthc. Mater. 2012, 1, 10–25. [Google Scholar] [CrossRef]
- Liao, I.C.; Chew, S.Y.; Leong, K.W. Aligned core-shell nanofibers delivering bioactive proteins. Nanomedicine (Lond.) 2006, 1, 465–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, H.; Hu, Y.; Li, Y.; Zhao, P.; Zhu, K.; Chen, W. A facile technique to prepare biodegradable coaxial electrospun nanofibers for controlled release of bioactive agents. J. Control. Release 2005, 108, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Dawson, E.; Mapili, G.; Erickson, K.; Taqvi, S.; Roy, K. Biomaterials for stem cell differentiation. Adv. Drug Deliv. Rev. 2008, 60, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Wahl, D.; Czernuszka, J. Collagen-hydroxyapatite composites for hard tissue repair. Eur. Cell Mater. 2006, 11, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Hur, W.; Kim, J.E.; Min, H.J.; Kim, S.; Min, H.S.; Kim, B.K.; Kim, S.H.; Choi, T.H.; Jung, Y. Self-assembling peptide nanofibers coupled with neuropeptide substance P for bone tissue engineering. Tissue Eng. Part A 2015, 21, 1237–1246. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Han, W.; Chen, H.; Tu, M.; Zeng, R.; Shi, Y.; Cha, Z.; Zhou, C. Preparation, structure and crystallinity of chitosan nano-fibers by a solid–liquid phase separation technique. Carbohydr. Polym. 2011, 83, 1541–1546. [Google Scholar] [CrossRef]
- Tang, Y.; Chen, L.; Zhao, K.; Wu, Z.; Wang, Y.; Tan, Q. Fabrication of PLGA/HA (core)-collagen/amoxicillin (shell) nanofiber membranes through coaxial electrospinning for guided tissue regeneration. Compos. Sci. Technol. 2016, 125, 100–107. [Google Scholar] [CrossRef]
- Wunner, F.M.; Bas, O.; Saidy, N.T.; Dalton, P.D.; Pardo, E.M.D.-J.; Hutmacher, D.W. Melt electrospinning writing of three-dimensional poly (ε-caprolactone) scaffolds with controllable morphologies for tissue engineering applications. Jove (J. Vis. Exp.) 2017, e56289. [Google Scholar] [CrossRef]
- Chakraborty, P.K.; Adhikari, J.; Saha, P. Facile fabrication of electrospun regenerated cellulose nanofiber scaffold for potential bone-tissue engineering application. Int. J. Biol. Macromol. 2019, 122, 644–652. [Google Scholar] [CrossRef]
- Yu, D.-G.; Li, X.-Y.; Wang, X.; Yang, J.-H.; Bligh, S.W.A.; Williams, G.R. Nanofibers fabricated using triaxial electrospinning as zero order drug delivery systems. ACS Appl. Mater. Interfaces 2015, 7, 18891–18897. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Li, W.; Yu, D.-G.; Wang, G.; Williams, G.R.; Zhang, Z. Tunable drug release from nanofibers coated with blank cellulose acetate layers fabricated using tri-axial electrospinning. Carbohydr. Polym. 2019, 203, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Gleeson, S.E.; Yu, T.; Khan, N.; Yucha, R.W.; Marcolongo, M.; Li, C.Y. Hierarchically ordered polymer nanofiber shish kebabs as a bone scaffold material. J. Biomed. Mater. Res. Part A 2017, 105, 1786–1798. [Google Scholar] [CrossRef] [PubMed]
- Rampichova, M.; Chvojka, J.; Jencova, V.; Kubikova, T.; Tonar, Z.; Erben, J.; Buzgo, M.; Dankova, J.; Litvinec, A.; Vocetkova, K.; et al. The combination of nanofibrous and microfibrous materials for enhancement of cell infiltration and in vivo bone tissue formation. Biomed. Mater. 2018, 13, 025004. [Google Scholar] [CrossRef] [PubMed]
- Taylor, G.I. Disintegration of water drops in an electric field. Proc. R. Soc. Lond. Ser. A Math. Phys. Sci. 1964, 280, 383–397. [Google Scholar] [CrossRef]
- Rutledge, G.C.; Fridrikh, S.V. Formation of fibers by electrospinning. Adv. Drug Deliv. Rev. 2007, 59, 1384–1391. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Yang, W.; Wang, T.; Mao, C.; Guo, L.; Xiao, J.; He, N. Coaxially electrospun core/shell structured poly (L-lactide) acid/chitosan nanofibers for potential drug carrier in tissue engineering. J. Biomed. Nanotechnol. 2013, 9, 1672–1678. [Google Scholar] [CrossRef]
- Gong, T.; Liu, T.; Zhang, L.; Ye, W.; Guo, X.; Wang, L.; Quan, L.; Pan, C. Design Redox-Sensitive Drug-Loaded Nanofibers for Bone Reconstruction. ACS Biomater. Sci. Eng. 2018, 4, 240–247. [Google Scholar] [CrossRef]
- Du, Z.; Zhang, S.; Liu, Y.; Zhao, J.; Lin, R.; Jiang, T. Facile fabrication of reticular polypyrrole–silicon core–shell nanofibers for high performance lithium storage. J. Mater. Chem. 2012, 22, 11636–11641. [Google Scholar] [CrossRef]
- Shao, W.; He, J.; Sang, F.; Ding, B.; Chen, L.; Cui, S.; Li, K.; Han, Q.; Tan, W. Coaxial electrospun aligned tussah silk fibroin nanostructured fiber scaffolds embedded with hydroxyapatite–tussah silk fibroin nanoparticles for bone tissue engineering. Mater. Sci. Eng. C 2016, 58, 342–351. [Google Scholar] [CrossRef]
- Shalumon, K.T.; Lai, G.-J.; Chen, C.-H.; Chen, J.-P. Modulation of bone-specific tissue regeneration by incorporating bone morphogenetic protein and controlling the shell thickness of silk fibroin/chitosan/nanohydroxyapatite core–shell nanofibrous membranes. ACS Appl. Mater. Interfaces 2015, 7, 21170–21181. [Google Scholar] [CrossRef]
- Yang, G.-Z.; Li, J.-J.; Yu, D.-G.; He, M.-F.; Yang, J.-H.; Williams, G.R. Nanosized sustained-release drug depots fabricated using modified tri-axial electrospinning. Acta Biomater. 2017, 53, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Mehrasa, M.; Asadollahi, M.A.; Ghaedi, K.; Salehi, H.; Arpanaei, A. Electrospun aligned PLGA and PLGA/gelatin nanofibers embedded with silica nanoparticles for tissue engineering. Int. J. Biol. Macromol. 2015, 79, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Madhurakkat Perikamana, S.K.; Lee, J.; Ahmad, T.; Jeong, Y.; Kim, D.-G.; Kim, K.; Shin, H. Effects of immobilized BMP-2 and nanofiber morphology on in vitro osteogenic differentiation of hMSCs and in vivo collagen assembly of regenerated bone. ACS Appl. Mater. Interfaces 2015, 7, 8798–8808. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Li, L.; Zhang, D.; Huang, S.; Jing, Z.; Wu, Y.; Zhao, Z.; Zhao, L.; Zhou, S. Incorporation of aligned PCL–PEG nanofibers into porous chitosan scaffolds improved the orientation of collagen fibers in regenerated periodontium. Acta Biomater. 2015, 25, 240–252. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, X.; Song, J.; Xu, X.; Xu, A.; Wang, M.; Xie, B.; Huang, E.; Deng, F.; Wei, S. Osteoinductive peptide-functionalized nanofibers with highly ordered structure as biomimetic scaffolds for bone tissue engineering. Int. J. Nanomed. 2015, 10, 7109. [Google Scholar]
- Anindyajati, A.; Boughton, P.; Ruys, A. The effect of rotating collector design on tensile properties and morphology of electrospun polycaprolactone fibres. MATEC Web Conf. 2015, 6, 02002. [Google Scholar] [CrossRef]
- He, X.; Xiao, Q.; Lu, C.; Wang, Y.; Zhang, X.; Zhao, J.; Zhang, W.; Zhang, X.; Deng, Y. Uniaxially aligned electrospun all-cellulose nanocomposite nanofibers reinforced with cellulose nanocrystals: Scaffold for tissue engineering. Biomacromolecules 2014, 15, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, E.S.; Mattoso, L.H.; Ito, E.N.; Gregorski, K.S.; Robertson, G.H.; Offeman, R.D.; Wood, D.F.; Orts, W.J.; Imam, S.H. Electrospun nanofibers of poly (vinyl alcohol) reinforced with cellulose nanofibrils. J. Biobased Mater. Bioenergy 2008, 2, 231–242. [Google Scholar] [CrossRef]
- Ma, J.; He, X.; Jabbari, E. Osteogenic differentiation of marrow stromal cells on random and aligned electrospun poly(l-lactide) nanofibers. Ann. Biomed. Eng. 2011, 39, 14–25. [Google Scholar] [CrossRef]
- Vasita, R.; Katti, D.S. Nanofibers and their applications in tissue engineering. Int. J. Nanomed. 2006, 1, 15–30. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, W.; Liu, S.; Wu, J.; Li, F.; Cao, L.; Liu, X.-d.; Mo, X.; Fan, C. Enhancement of chondrogenic differentiation of rabbit mesenchymal stem cells by oriented nanofiber yarn-collagen type I/hyaluronate hybrid. Mater. Sci. Eng. C 2016, 58, 1071–1076. [Google Scholar] [CrossRef] [PubMed]
- Mi, H.Y.; Salick, M.R.; Jing, X.; Crone, W.C.; Peng, X.F.; Turng, L.S. Electrospinning of unidirectionally and orthogonally aligned thermoplastic polyurethane nanofibers: Fiber orientation and cell migration. J. Biomed. Mater. Res. A 2015, 103, 593–603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, B.; Yan, X.; He, H.-W.; Yu, M.; Ning, X.; Long, Y.-Z. Solvent-free electrospinning: Opportunities and challenges. Polym. Chem. 2017, 8, 333–352. [Google Scholar] [CrossRef]
- Holzwarth, J.M.; Ma, P.X. Biomimetic nanofibrous scaffolds for bone tissue engineering. Biomaterials 2011, 32, 9622–9629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, K.E.; Kim, B.S.; Kim, M.H.; You, H.K.; Lee, J.; Park, W.H. Basic fibroblast growth factor-encapsulated PCL nano/microfibrous composite scaffolds for bone regeneration. Polymer 2015, 76, 8–16. [Google Scholar] [CrossRef]
- Kim, B.S.; Park, K.E.; Kim, M.H.; You, H.K.; Lee, J.; Park, W.H. Effect of nanofiber content on bone regeneration of silk fibroin/poly (ε-caprolactone) nano/microfibrous composite scaffolds. Int. J. Nanomed. 2015, 10, 485. [Google Scholar]
- Abdal-hay, A.; Abbasi, N.; Gwiazda, M.; Hamlet, S.; Ivanovski, S. Novel polycaprolactone/hydroxyapatite nanocomposite fibrous scaffolds by direct melt-electrospinning writing. Eur. Polym. J. 2018, 105, 257–264. [Google Scholar] [CrossRef] [Green Version]
- Manakhov, A.; Kedroňová, E.; Medalová, J.; Černochová, P.; Obrusník, A.; Michlíček, M.; Shtansky, D.V.; Zajíčková, L. Carboxyl-anhydride and amine plasma coating of PCL nanofibers to improve their bioactivity. Mater. Des. 2017, 132, 257–265. [Google Scholar] [CrossRef]
- Aragon, J.; Navascues, N.; Mendoza, G.; Irusta, S. Laser-treated electrospun fibers loaded with nano-hydroxyapatite for bone tissue engineering. Int. J. Pharm. 2017, 525, 112–122. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Lim, S.; Birajdar, M.; Lee, S.-H.; Park, H. Fabrication of FGF-2 immobilized electrospun gelatin nanofibers for tissue engineering. Int. J. Biol. Macromol. 2016, 93, 1559–1566. [Google Scholar] [CrossRef]
- Chahal, S.; Hussain, F.S.J.; Kumar, A.; Yusoff, M.M.; Rasad, M.S.B.A. Electrospun hydroxyethyl cellulose nanofibers functionalized with calcium phosphate coating for bone tissue engineering. RSC Adv. 2015, 5, 29497–29504. [Google Scholar] [CrossRef] [Green Version]
- Luo, X.; Guo, Z.; He, P.; Chen, T.; Li, L.; Ding, S.; Li, H. Study on structure, mechanical property and cell cytocompatibility of electrospun collagen nanofibers crosslinked by common agents. Int. J. Biol. Macromol. 2018, 113, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Siow, K.S.; Britcher, L.; Kumar, S.; Griesser, H.J. Plasma methods for the generation of chemically reactive surfaces for biomolecule immobilization and cell colonization—A review. Plasma Process. Polym. 2006, 3, 392–418. [Google Scholar] [CrossRef]
- Chen, J.-P.; Su, C.-H. Surface modification of electrospun PLLA nanofibers by plasma treatment and cationized gelatin immobilization for cartilage tissue engineering. Acta Biomater. 2011, 7, 234–243. [Google Scholar] [CrossRef]
- Ho, M.-H.; Hou, L.-T.; Tu, C.-Y.; Hsieh, H.-J.; Lai, J.-Y.; Chen, W.-J.; Wang, D.-M. Promotion of Cell affinity of porous PLLA scaffolds by immobilization of RGD peptides via plasma treatment. Macromol. Biosci. 2006, 6, 90–98. [Google Scholar] [CrossRef]
- Sanders, J.E.; Lamont, S.E.; Karchin, A.; Golledge, S.L.; Ratner, B.D. Fibro-porous meshes made from polyurethane micro-fibers: Effects of surface charge on tissue response. Biomaterials 2005, 26, 813–818. [Google Scholar] [CrossRef]
- Park, C.; Xue, R.; Lannutti, J.J.; Farson, D.F. Ablation characteristics of electrospun core-shell nanofiber by femtosecond laser. Mater. Sci. Eng. C 2016, 65, 232–239. [Google Scholar] [CrossRef]
- Yano, T.; Yah, W.O.; Yamaguchi, H.; Terayama, Y.; Nishihara, M.; Kobayashi, M.; Takahara, A. Precise control of surface physicochemical properties for electrospun fiber mats by surface-initiated radical polymerization. Polym. J. 2011, 43, 838–848. [Google Scholar] [CrossRef] [Green Version]
- Bart, J.; Tiggelaar, R.; Yang, M.; Schlautmann, S.; Zuilhof, H.; Gardeniers, H. Room-temperature intermediate layer bonding for microfluidic devices. Lab. A Chip. 2009, 9, 3481–3488. [Google Scholar] [CrossRef]
- Park, Y.J.; Kim, K.H.; Lee, J.Y.; Ku, Y.; Lee, S.J.; Min, B.M.; Chung, C.P. Immobilization of bone morphogenetic protein-2 on a nanofibrous chitosan membrane for enhanced guided bone regeneration. Biotechnol. Appl. Biochem. 2006, 43, 17–24. [Google Scholar]
- Shelke, N.B.; James, R.; Laurencin, C.T.; Kumbar, S.G. Polysaccharide biomaterials for drug delivery and regenerative engineering. Polym. Adv. Technol. 2014, 25, 448–460. [Google Scholar] [CrossRef]
- Croll, T.I.; O’Connor, A.J.; Stevens, G.W.; Cooper-White, J.J. Controllable surface modification of poly (lactic-co-glycolic acid)(PLGA) by hydrolysis or aminolysis I: Physical, chemical, and theoretical aspects. Biomacromolecules 2004, 5, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Wang, Y.; Sun, T.; Wang, B.; Zhang, H. Bioinspired surface functionalization for improving osteogenesis of electrospun polycaprolactone nanofibers. Langmuir 2018, 34, 15544–15550. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Xiong, D. Friction properties of nano-hydroxyapatite reinforced poly (vinyl alcohol) gel composites as an articular cartilage. Wear 2009, 266, 699–703. [Google Scholar] [CrossRef]
- Shakir, M.; Jolly, R.; Khan, M.S.; e Iram, N.; Khan, H.M. Nano-hydroxyapatite/chitosan–starch nanocomposite as a novel bone construct: Synthesis and in vitro studies. Int. J. Biol. Macromol. 2015, 80, 282–292. [Google Scholar] [CrossRef]
- Lee, J.S.; Baek, S.D.; Venkatesan, J.; Bhatnagar, I.; Chang, H.K.; Kim, H.T.; Kim, S.-K. In vivo study of chitosan-natural nano hydroxyapatite scaffolds for bone tissue regeneration. Int. J. Biol. Macromol. 2014, 67, 360–366. [Google Scholar] [CrossRef]
- Ren, K.; Wang, Y.; Sun, T.; Yue, W.; Zhang, H. Electrospun PCL/gelatin composite nanofiber structures for effective guided bone regeneration membranes. Mater. Sci. Eng. C 2017, 78, 324–332. [Google Scholar] [CrossRef]
- Frohbergh, M.E.; Katsman, A.; Botta, G.P.; Lazarovici, P.; Schauer, C.L.; Wegst, U.G.K.; Lelkes, P.I. Electrospun hydroxyapatite-containing chitosan nanofibers crosslinked with genipin for bone tissue engineering. Biomaterials 2012, 33, 9167–9178. [Google Scholar] [CrossRef] [Green Version]
- Çay, A.; Akçakoca Kumbasar, E.P.; Keskin, Z.; Akduman, Ç.; Şendemir Ürkmez, A. Crosslinking of poly(vinyl alcohol) nanofibres with polycarboxylic acids: Biocompatibility with human skin keratinocyte cells. J. Mater. Sci. 2017, 52, 12098–12108. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, T.; Hua, W.; Li, P.; Wang, X. 3D Porous poly(lactic acid)/regenerated cellulose composite scaffolds based on electrospun nanofibers for biomineralization. Colloids Surf. A Physicochem. Eng. Asp. 2019, 585, 124048. [Google Scholar] [CrossRef]



| Developments | Advantages | Limitations | Example of Recent Developments |
|---|---|---|---|
| Conventional electrospinning | - Facile and versatile method | - Non-patterned products - Lack of tensile strength | - Solvent system developed for high porosity fiber [39] |
| Multi-axial electrospinning | - Core-shell structure - Permits various materials to be immobilized, good for drug delivery - “Lubricant effect” prevents clog | - Toxic solvent - Poor cell infiltration | - Functional trilayer nanofibers for zero-order drug delivery [40] - Prevents jet instability by triaxial spinneret [41] |
| Electrospinning with a modified collector and high-speed rotation | - Aligned structure - Guides oriented arrangement and elongation of cells - Decrease in diameter - Good mechanical properties | - Toxic solvent - Complex setup - Clogging or jet instability can occur | - Hierarchically aligned polymer nanofiber as a bone scaffold [42] |
| Melt-electrospinning | - Three-dimensional structure - Larger pore size - Diverse diameter range - Eco-friendly method | - Cost for an extra instrument - Mostly amorphous fiber and thermal degradation | - Combination of nano- and micro-fibrous scaffolds for enhancing cell infiltration and bone tissue formation [43] |
| Developments | Advantages | Limitations | Example of Recent Developments |
|---|---|---|---|
| Plasma and laser treatment | - Improve surface hydrophilicity - Increase porosity - Increase cell adhesion and proliferation rate in fibroblast cells | - Fast degradation of functional groups on surface | - Plasma polymerization increases the density of functional groups [68] - Laser ablation on PCL/PVAc loaded hydroxyapatite [69] |
| Surface functionalization | - Strong bond, difficult to break - Diversity of functional groups - Provides delivery function | - Influencing the mechanical properties of the fiber - Batch-to-batch inconsistency | - Growth factor immobilization on gelatin nanofiber by avidin-biotin conjugation [70] |
| Inorganic combination | - Improve mechanical properties - Induces bone formation | - Compromising the porosity | - Bone-like calcium phosphate deposition onto cellulose fibers [71] |
| Cross-linking method | - Improved mechanical properties - Enhanced biodegradation time | - Cytotoxicity problem - Non-oriented structure | - Low-cytotoxicity crosslinking of nanofiber by the natural compound, genipin [72] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Udomluck, N.; Koh, W.-G.; Lim, D.-J.; Park, H. Recent Developments in Nanofiber Fabrication and Modification for Bone Tissue Engineering. Int. J. Mol. Sci. 2020, 21, 99. https://doi.org/10.3390/ijms21010099
Udomluck N, Koh W-G, Lim D-J, Park H. Recent Developments in Nanofiber Fabrication and Modification for Bone Tissue Engineering. International Journal of Molecular Sciences. 2020; 21(1):99. https://doi.org/10.3390/ijms21010099
Chicago/Turabian StyleUdomluck, Nopphadol, Won-Gun Koh, Dong-Jin Lim, and Hansoo Park. 2020. "Recent Developments in Nanofiber Fabrication and Modification for Bone Tissue Engineering" International Journal of Molecular Sciences 21, no. 1: 99. https://doi.org/10.3390/ijms21010099
APA StyleUdomluck, N., Koh, W. -G., Lim, D. -J., & Park, H. (2020). Recent Developments in Nanofiber Fabrication and Modification for Bone Tissue Engineering. International Journal of Molecular Sciences, 21(1), 99. https://doi.org/10.3390/ijms21010099