Does the Urothelium of Old Mice Regenerate after Chitosan Injury as Quickly as the Urothelium of Young Mice?
Abstract
:1. Introduction
2. Results
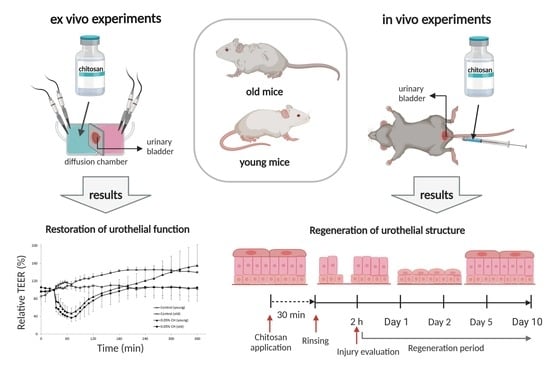
2.1. Restoration of Urothelial Function in Ex Vivo Experiments
2.2. Results of In Vivo Experiments
2.2.1. Morphological Evaluation of Urothelial Injury in Young and Old Mice
2.2.2. Restoration of Urothelial Structure after Chitosan Treatment in Young Mice
2.2.3. Restoration of Urothelial Structure after Chitosan Treatment in Old Mice
2.2.4. Proliferative and Apoptotic Activity of the Urothelium after Chitosan Treatment in Young and Old Mice
2.2.5. Urothelial Differentiation after Chitosan Treatment in Young and Old Mice
Expression of Uroplakins
Expression of Cytokeratin 20
2.2.6. Acute Inflammation in the Bladder Wall after Chitosan Treatment in Young and Old Mice
Histological Evaluation of the Bladder Edema
Neutrophil Infiltration
Expression of Cyclooxygenase 2 (Cox-2) and Serum Amyloid A3 (Saa3)
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Ex Vivo Experiments
4.3. In Vivo Experiments
4.4. Preparation of Paraffin Sections and Cryosections
4.4.1. Immunolabeling on Paraffin Sections
4.4.2. Immunofluorescence Labeling on Cryosections
4.5. Analysis of Proliferative and Apoptotic Activity
4.6. Transmission Electron Microscopy (TEM)
4.7. Scanning Electron Microscopy (SEM)
4.8. Semi-Quantitative Assessment of Acute Inflammation in the Urinary Bladder Wall
4.8.1. Measurement of the Relative Thickness of the Lamina Propria
4.8.2. Analysis of Neutrophil Infiltration
4.9. RNA Isolation and Quantitative PCR Analysis
4.10. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AI | apoptotic index |
| BSA | bovine serum albumin |
| CH | chitosan |
| CK20 | cytokeratin 20 |
| Cox-2 | cyclooxygenase 2 |
| DAB | 3,3′-diaminobenzidine |
| DAPI | 4′,6-diamidino-2-phenylindole |
| FCS | fetal calf serum |
| PBS | Phosphate-buffered saline |
| PI | proliferative index |
| rTh | relative thickness of lamina propria |
| Saa3 | serum amyloid A3 |
| SEM | scanning electron microscopy |
| TEER | transepithelial electrical resistance |
| TEM | transmission electron microscopy |
References
- Ruben, F.L.; Dearwater, S.R.; Norden, C.W.; Kuller, L.H.; Gartner, K.; Shalley, A.; Warshafsky, G.; Kelsey, S.F.; O’donnell, C.; Means, E.; et al. Clinical Infections in the Noninstitutionalized Geriatric Age Group: Methods Utilized and Incidence of Infections: The Pittsburgh Good Health Study. Am. J. Epidemiol. 1995, 141, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Foxman, B. The Epidemiology of Urinary Tract Infection. Nat. Rev. Urol. 2010, 7, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.M.; Lowder, J.L. Diagnosis and Treatment of Urinary Tract Infections across Age Groups. Am. J. Obstet. Gynecol. 2018, 219, 40–51. [Google Scholar] [CrossRef]
- Erman, A.; Hergouth, V.K.; Blango, M.G.; Kos, M.K.; Mulvey, M.A.; Veranič, P. Repeated Treatments with Chitosan in Combination with Antibiotics Completely Eradicate Uropathogenic Escherichia Coli from Infected Mouse Urinary Bladders. J. Infect. Dis. 2017, 216, 375–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romih, R.; Koprivec, D.; Martincic, D.S.; Jezernik, K. Restoration of the Rat Urothelium after Cyclophosphamide Treatment. Cell Biol. Int. 2001, 25, 531–537. [Google Scholar] [CrossRef]
- Jezernik, K.; Romih, R.; Mannherz, H.G.; Koprivec, D. Immunohistochemical Detection of Apoptosis, Proliferation and Inducible Nitric Oxide Synthase in Rat Urothelium Damaged by Cyclophosphamide Treatment. Cell Biol. Int. 2003, 27, 863–869. [Google Scholar] [CrossRef]
- Veranič, P.; Erman, A.; Kerec-Kos, M.; Bogataj, M.; Mrhar, A.; Jezernik, K. Rapid Differentiation of Superficial Urothelial Cells after Chitosan-Induced Desquamation. Histochem. Cell Biol. 2009, 131, 129–139. [Google Scholar] [CrossRef]
- Erman, A.; Kerec Kos, M.; Žakelj, S.; Resnik, N.; Romih, R.; Veranič, P. Correlative Study of Functional and Structural Regeneration of Urothelium after Chitosan-Induced Injury. Histochem. Cell Biol. 2013, 140, 521–531. [Google Scholar] [CrossRef]
- Hicks, R.M. The Mammalian Urinary Bladder: An Accommodating Organ. Biol. Rev. Camb. Philos. Soc. 1975, 50, 215–246. [Google Scholar] [CrossRef]
- Apodaca, G. The Uroepithelium: Not Just a Passive Barrier. Traffic 2004, 5, 117–128. [Google Scholar] [CrossRef]
- Kachar, B.; Liang, F.; Lins, U.; Ding, M.; Wu, X.-R.; Stoffler, D.; Aebi, U.; Sun, T.-T. Three-Dimensional Analysis of the 16 Nm Urothelial Plaque Particle: Luminal Surface Exposure, Preferential Head-to-Head Interaction, and Hinge Formation 1 1Edited by W. Baumeisser. J. Mol. Biol. 1999, 285, 595–608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romih, R.; Veranič, P.; Jezernik, K. Actin Filaments during Terminal Differentiation of Urothelial Cells in the Rat Urinary Bladder. Histochem. Cell Biol. 1999, 112, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Veranič, P.; Romih, R.; Jezernik, K. What Determines Differentiation of Urothelial Umbrella Cells? Eur. J. Cell Biol. 2004, 83, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Hudoklin, S.; Jezernik, K.; Neumüller, J.; Pavelka, M.; Romih, R. Urothelial Plaque Formation in Post-Golgi Compartments. PLoS ONE 2011, 6, e23636. [Google Scholar] [CrossRef] [Green Version]
- Lewis, S.A.; Diamond, J.M. Na+ Transport by Rabbit Urinary Bladder, a Tight Epithelium. J. Membr. Biol. 1976, 28, 1–40. [Google Scholar] [CrossRef]
- Negrete, H.O.; Lavelle, J.P.; Berg, J.; Lewis, S.A.; Zeidel, M.L. Permeability Properties of the Intact Mammalian Bladder Epithelium. Am. J. Physiol. 1996, 271, F886–F894. [Google Scholar] [CrossRef]
- Jost, S.P.; Potten, C.S. Urothelial Proliferation In Growing Mice. Cell Prolif. 1986, 19, 155–160. [Google Scholar] [CrossRef]
- Jost, S.P.; Gosling, J.A.; Dixon, J.S. The Morphology of Normal Human Bladder Urothelium. J. Anat. 1989, 167, 103–115. [Google Scholar]
- Martin, B.F. Cell Replacement and Differentiation in Transitional Epithelium: A Histological and Autoradiographic Study of the Guinea-Pig Bladder and Ureter. J. Anat. 1972, 112, 433–455. [Google Scholar]
- Jacob, J.; Hindmarsh, J.R.; Ludgate, C.M.; Chisholm, G.D. Observations on the Ultrastructure of Human Urothelium: The Response of Normal Bladder of Elderly Subjects to Hyperthermia. Urol. Res. 1982, 10, 227–237. [Google Scholar] [CrossRef]
- Watanabe, S.; Sasaki, J. 12-O-Tetradecanoylphorbol-13-Acetate Induces Selective Desquamation of Superficial Cells in Rat Urinary Bladder Epithelium. Cell Tissue Res. 1992, 268, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Kos, M.K.; Veranič, P.; Erman, A. Poly-L-Lysine as an Effective and Safe Desquamation Inducer of Urinary Bladder Epithelium. Polymers 2019, 11, 1506. [Google Scholar] [CrossRef] [Green Version]
- Kos, M.K.; Bogataj, M.; Veranič, P.; Mrhar, A. Permeability of Pig Urinary Bladder Wall: Time and Concentration Dependent Effect of Chitosan. Biol. Pharm. Bull. 2006, 29, 1685–1691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bower, J.M.; Eto, D.S.; Mulvey, M.A. Covert Operations of Uropathogenic Escherichia Coli within the Urinary Tract. Traffic 2005, 6, 18–31. [Google Scholar] [CrossRef]
- Chamie, K.; Litwin, M.S.; Bassett, J.C.; Daskivich, T.J.; Lai, J.; Hanley, J.M.; Konety, B.R.; Saigal, C.S. Recurrence of High-Risk Bladder Cancer: A Population-Based Analysis. Cancer 2013, 119, 3219–3227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Highton, A.J.; Girardin, A.; Bell, G.M.; Hook, S.M.; Kemp, R.A. Chitosan Gel Vaccine Protects against Tumour Growth in an Intracaecal Mouse Model of Cancer by Modulating Systemic Immune Responses. BMC Immunol. 2016, 17, 39. [Google Scholar] [CrossRef] [Green Version]
- Navarro, S.; Driscoll, B. Regeneration of the Aging Lung: A Mini-Review. Gerontology 2017, 63, 270–280. [Google Scholar] [CrossRef]
- Sousounis, K.; Baddour, J.A.; Tsonis, P.A. Aging and Regeneration in Vertebrates. Curr. Top. Dev. Biol. 2014, 108, 217–246. [Google Scholar] [CrossRef]
- Pibiri, M. Liver Regeneration in Aged Mice: New Insights. Aging 2018, 10, 1801–1824. [Google Scholar] [CrossRef]
- Kaş, H.S. Chitosan: Properties, Preparations and Application to Microparticulate Systems. J. Microencapsul. 1997, 14, 689–711. [Google Scholar] [CrossRef]
- Koide, S.S. Chitin-Chitosan: Properties, Benefits and Risks. Nutr. Res. 1998, 18, 1091–1101. [Google Scholar] [CrossRef]
- Singla, A.K.; Chawla, M. Chitosan: Some Pharmaceutical and Biological Aspects-an Update. J. Pharm. Pharmacol. 2001, 53, 1047–1067. [Google Scholar] [CrossRef]
- Hejazi, R.; Amiji, M. Chitosan-Based Gastrointestinal Delivery Systems. J. Control. Release 2003, 89, 151–165. [Google Scholar] [CrossRef]
- Hari-Dass, R.; Shah, C.; Meyer, D.J.; Raynes, J.G. Serum Amyloid A Protein Binds to Outer Membrane Protein A of Gram-Negative Bacteria. J. Biol. Chem. 2005, 280, 18562–18567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seibert, K.; Masferrer, J.L. Role of Inducible Cyclooxygenase (COX-2) in Inflammation. Receptor 1994, 4, 17–23. [Google Scholar] [PubMed]
- DuBois, R.N.; Abramson, S.B.; Crofford, L.; Gupta, R.A.; Simon, L.S.; Van De Putte, L.B.A.; Lipsky, P.E. Cyclooxygenase in Biology and Disease. FASEB J. 1998, 12, 1063–1073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ranaldi, G.; Marigliano, I.; Vespignani, I.; Perozzi, G.; Sambuy, Y. The Effect of Chitosan and Other Polycations on Tight Junction Permeability in the Human Intestinal Caco-2 Cell Line (1). J. Nutr. Biochem. 2002, 13, 157–167. [Google Scholar] [CrossRef]
- Cohen, S.M.; Cano, M.; Sakata, T.; Johansson, S.L. Ultrastructural Characteristics of the Fetal and Neonatal Rat Urinary Bladder. Scanning Microsc. 1988, 2, 2091–2104. [Google Scholar]
- Ayres, P.H.; Shinohara, Y.; Frith, C.H. Morphological Observations on the Epithelium of the Developing Urinary Bladder of the Mouse and Rat. J. Urol. 1985, 133, 506–512. [Google Scholar] [CrossRef]
- Santos, C.P.; Lapi, E.; Martínez de Villarreal, J.; Álvaro-Espinosa, L.; Fernández-Barral, A.; Barbáchano, A.; Domínguez, O.; Laughney, M.A.; Megías, D.; Muñoz, A.; et al. Urothelial organoids originating from Cd49fhigh mouse stem cells display Notch-dependent differentiation capacity. Nat. Commun. 2019, 10, 4407. [Google Scholar] [CrossRef] [Green Version]
- Koss, L.G. A Light and Electron Microscopic Study of the Effects of a Single Dose of Cyclophosphamide on Various Organs in the Rat. I. The Urinary Bladder. Lab. Invest. 1967, 16, 44–65. [Google Scholar] [PubMed]
- Romih, R.; Jezernik, K.; Mašera, A. Uroplakins and Cytokeratins in the Regenerating Rat Urothelium after Sodium Saccharin Treatment. Histochem. Cell Biol. 1998, 109, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.; Lee, J.; Guo, N.; Kim, J.; Lim, A.; Qu, L.; Mysorekar, I.U.; Beachy, P.A. Hedgehog/Wnt Feedback Supports Regenerative Proliferation of Epithelial Stem Cells in Bladder. Nature 2011, 472, 110–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colopy, S.A.; Bjorling, D.E.; Mulligan, W.A.; Bushman, W. A Population of Progenitor Cells in the Basal and Intermediate Layers of the Murine Bladder Urothelium Contributes to Urothelial Development and Regeneration. Dev. Dyn. 2014, 243, 988–998. [Google Scholar] [CrossRef] [Green Version]
- Perše, M.; Injac, R.; Erman, A. Oxidative Status and Lipofuscin Accumulation in Urothelial Cells of Bladder in Aging Mice. PLoS ONE 2013, 8, e59638. [Google Scholar] [CrossRef]
- Schmitz, G.; Muller, G. Structure and Function of Lamellar Bodies, Lipid-Protein Complexes Involved in Storage and Secretion of Cellular Lipids. J. Lipid Res. 1991, 32, 1539–1570. [Google Scholar]
- Reasor, M.J. A Review of the Biology and Toxicologic Implications of the Induction of Lysosomal Lamellar Bodies by Drugs. Toxicol. Appl. Pharmacol. 1989, 97, 47–56. [Google Scholar] [CrossRef]
- Hostetler, K.Y. Molecular Studies of the Induction of Cellular Phospholipidosis by Cationic Amphiphilic Drugs. Fed. Proc. 1984, 43, 2582–2585. [Google Scholar]
- Cuervo, A.M.; Dice, J.F. Age-Related Decline in Chaperone-Mediated Autophagy. J. Biol. Chem. 2000, 275, 31505–31513. [Google Scholar] [CrossRef] [Green Version]
- Del Roso, A.; Vittorini, S.; Cavallini, G.; Donati, A.; Gori, Z.; Masini, M.; Pollera, M.; Bergamini, E. Ageing-Related Changes in the in Vivo Function of Rat Liver Macroautophagy and Proteolysis. Exp. Gerontol. 2003, 38, 519–527. [Google Scholar] [CrossRef]
- Terman, A. The Effect of Age on Formation and Elimination of Autophagic Vacuoles in Mouse Hepatocytes. Gerontology 1995, 41, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Tate, T.; Batourina, E.; Truschel, S.T.; Potter, S.; Adam, M.; Xiang, T.; Picard, M.; Reiley, M.; Schneider, K.; et al. Pparg promotes differentiation and regulates mitochondrial gene expression in bladder epithelial cells. Nat. Commun. 2019, 10, 4589. [Google Scholar] [CrossRef] [PubMed]
- Kisilevsky, R. Serum Amyloid A (SAA), a Protein without a Function: Some Suggestions with Reference to Cholesterol Metabolism. Med. Hypotheses 1991, 35, 337–341. [Google Scholar] [CrossRef]
- Erman, A.; Lakota, K.; Mrak-Poljsak, K.; Blango, M.G.; Krizan-Hergouth, V.; Mulvey, M.A.; Sodin-Semrl, S.; Veranic, P. Uropathogenic Escherichia Coli Induces Serum Amyloid a in Mice Following Urinary Tract and Systemic Inoculation. PLoS ONE 2012, 7, e32933. [Google Scholar] [CrossRef] [Green Version]













| Neutrophil Infiltration | ||||
|---|---|---|---|---|
| Time Points after Chitosan Treatment | Abundance (Score Value 1) | Distribution (Score Value 2) | Sum of Score Values 1 and 2 | |
| Young mice | Control | 0 | 0 | 0 |
| 2 h | 3.5 | 2.5 | 6 | |
| Day 1 | 3.5 | 3.5 | 7 | |
| Day 2 | 3.5 | 3.5 | 7 | |
| Day 5 | 1 | 1 | 2 | |
| Day 10 | 0.5 | 0.5 | 1 | |
| Old mice | Control | 0 | 0 | 0 |
| 2 h | 2 | 2.5 | 4.5 | |
| Day 1 | 4 | 2.5 | 6.5 | |
| Day 2 | 0.5 | 1.5 | 2 | |
| Day 5 | 0.5 | 0.5 | 1 | |
| Day 10 | 0.5 | 1.5 | 2 | |
| Primary Antibodies | Secondary Antibodies |
|---|---|
| Anti-Ki-67 antigen (1:100; rabbit monoclonal/SP6; Abcam, Cambridge, UK) | Swine anti-rabbit (1:200; biotinylated, Dako, Glostrup, Denmark) or Goat anti-rabbit (1:200; Alexa Fluor® 555; Invitrogen, Carlsbad, CA, USA) |
| Anti-uroplakins (AUM) (1:10,000; rabbit polyclonal; courtesy of T.T. Sun from New York University, New York, NY, USA) | Goat anti-rabbit (1:400; biotinylated, Dako, Glostrup, Denmark) |
| Anti-neutrophils (1:100; rat monoclonal/NIMP-R14; Abcam, Cambridge, UK) | Donkey anti-rat (1:300; Alexa Fluor® 488; Invitrogen, Carlsbad, CA, USA) |
| Anti-active caspase 3 (1:10; rabbit polyclonal; Abcam, Cambridge, UK) | Goat anti-rabbit (1:400; Alexa Fluor® 488; Invitrogen, Carlsbad, CA, USA) |
| Primary Antibodies | Secondary Antibodies |
|---|---|
| Anti-cytokeratin 20 (1:50; mouse monoclonal/Ks20.8; Dako, Glostrup, Denmark) | Goat anti-mouse (1:200; Alexa Fluor® 488; Invitrogen, Carlsbad, CA, USA) |
| Anti-neutrophils (1:100; rat monoclonal/NIMP-R14; Abcam, Cambridge, UK) | Donkey anti-rat (1:300; Alexa Fluor® 488; Invitrogen, Carlsbad, CA, USA) |
| Anti-Ki-67 antigen (1:200; rabbit monoclonal/SP6; Abcam, Cambridge, UK) | Goat anti-rabbit (1:400; Alexa Fluor® 555; Invitrogen, Carlsbad, CA, USA) |
| Anti-active caspase-3 (1:200; rabbit polyclonal; Abcam, Cambridge, UK) | Goat anti-rabbit (1:400; Alexa Fluor® 488; Invitrogen, Carlsbad, CA, USA) |
| Anti-collagen IV (1:300; rabbit polyclonal; Abcam, Cambridge, UK) | Goat anti-rabbit (1:400; Alexa Fluor® 555; Invitrogen, Carlsbad, CA, USA) |
| Abundance | Score Value 1 | Distribution | Score Value 2 | |
|---|---|---|---|---|
| 0 | 0 | – | 0 | |
| ≤10 | 1 | Lamina propria | 1 | |
| 10 < x < 50 | 2 | Urothelium + lamina propria | 2 | |
| 50–100 Countless | 3 4 | Urothelium + lamina propria + muscle layer (transmural) | 3 | |
| Sum of score values 1 and 2: 0–7 | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erzar, E.; Kerec Kos, M.; Lakota, K.; Veranič, P.; Erman, A. Does the Urothelium of Old Mice Regenerate after Chitosan Injury as Quickly as the Urothelium of Young Mice? Int. J. Mol. Sci. 2020, 21, 3502. https://doi.org/10.3390/ijms21103502
Erzar E, Kerec Kos M, Lakota K, Veranič P, Erman A. Does the Urothelium of Old Mice Regenerate after Chitosan Injury as Quickly as the Urothelium of Young Mice? International Journal of Molecular Sciences. 2020; 21(10):3502. https://doi.org/10.3390/ijms21103502
Chicago/Turabian StyleErzar, Eva, Mojca Kerec Kos, Katja Lakota, Peter Veranič, and Andreja Erman. 2020. "Does the Urothelium of Old Mice Regenerate after Chitosan Injury as Quickly as the Urothelium of Young Mice?" International Journal of Molecular Sciences 21, no. 10: 3502. https://doi.org/10.3390/ijms21103502
APA StyleErzar, E., Kerec Kos, M., Lakota, K., Veranič, P., & Erman, A. (2020). Does the Urothelium of Old Mice Regenerate after Chitosan Injury as Quickly as the Urothelium of Young Mice? International Journal of Molecular Sciences, 21(10), 3502. https://doi.org/10.3390/ijms21103502