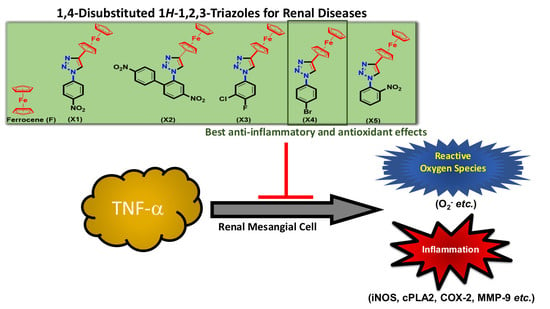
1,4-Disubstituted 1H-1,2,3-Triazoles for Renal Diseases: Studies of Viability, Anti-Inflammatory, and Antioxidant Activities
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization
X-ray Crystal Structure
2.2. In Vitro Characterizations
2.2.1. Antioxidant Effect on RMCs
2.2.2. Effect on iNOS Expression and NO Production in RMCs
2.2.3. Effect on the Expression of Inflammatory Proteins in RMCs
2.3. In Silico Studies
2.3.1. ADMET Predictions
2.3.2. Docking Results
3. Materials and Methods
3.1. Synthesis and Characterization
3.2. In Vitro Characterizations of Synthesized Compounds
3.2.1. Cell Culture in the Presence of Ferrocene-1H-1,2,3-Triazole Hybrids
3.2.2. Viability Assay
3.2.3. Gelatin Zymography
3.2.4. Nitrite Production
3.2.5. Western Blotting for Inflammatory Proteins
3.2.6. Real-time PCR
3.2.7. cPLA2 and COX-2 Enzyme Activity
3.3. In Silico Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| MMP-9 | Matrix metalloproteinase-9 |
| PGE2 | Prostaglandin E2 |
| cPLA2 | Cytosolic phospholipase A2 |
| TNF-α | Tumor necrosis factor-α |
| MCs | Mesangial cells |
| NSAIDs | Nonsteroidal anti-inflammatory drugs |
| NOS | NO synthase |
| TIMPs | Tissue inhibitors of metalloproteinases |
| iNOS | Inducible nitric oxide synthase |
References
- Garavito, R.M. The cyclooxygenase-2 stucture: New drugs for an old target? Nat. Struct. Biol. 1996, 3, 897–901. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, J.; Gai, Z.; Kullak-Ublick, G.A.; Liu, Z. TNF-α deficiency prevents renal inflammation and oxidative stress in obese mice. Kidney Blood Press. Res. 2017, 42, 416–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yung, S.; Cheung, K.F.; Zhang, Q.; Chan, T.M. Mediators of inflammation and their effect on resident renal cells: Implications in lupus nephritis. Clin. Dev. Immunol. 2013, 2013, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, I.-T.; Lin, C.-F.; Huang, Y.-L.; Chong, K.-Y.; Hsieh, M.-F.; Huang, T.-H.; Cheng, C.-Y. Protective mechanisms of resveratrol derivatives against TNF-α-induced inflammatory responses in rat mesangial cells. Cytokine 2019, 113, 380–392. [Google Scholar] [CrossRef]
- Sato, T.; Van Dixhoorn, M.; Heemskerk, E.; Van Es, L.; Daha, M. C1q, a subunit of the first component of complement, enhances antibody-mediated apoptosis of cultured rat glomerular mesangial cells. Clin. Exp. Immunol. 1997, 109, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Narula, S.; Tandon, C.; Tandon, S. Role of matrix metalloproteinases in degenerative kidney disorders. Curr. Med. Chem. 2018, 25, 1805–1816. [Google Scholar] [CrossRef] [PubMed]
- Luiking, Y.C.; Engelen, M.P.; Deutz, N.E. Regulation of nitric oxide production in health and disease. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 97–104. [Google Scholar] [CrossRef] [Green Version]
- Fiorucci, S.; Meli, R.; Bucci, M.; Cirino, G. Dual inhibitors of cyclooxygenase and 5-lipoxygenase. A new avenue in anti-inflammatory therapy? Biochem. Pharmacol. 2001, 62, 1433–1438. [Google Scholar] [CrossRef]
- Haque, A.; Hsieh, M.-F.; Hassan, S.I.; Faizi, M.S.H.; Saha, A.; Dege, N.; Rather, J.A.; Khan, M.S. Synthesis, characterization, and pharmacological studies of ferrocene-1H-1, 2, 3-triazole hybrids. J. Mol. Struct. 2017, 1146, 536–545. [Google Scholar] [CrossRef]
- Kolb, H.C.; Sharpless, K.B. The growing impact of click chemistry on drug discovery. Drug Discov. Today 2003, 8, 1128–1137. [Google Scholar] [CrossRef]
- Guo, W.-Y.; Chen, L.-Z.; Shen, B.-N.; Liu, X.-H.; Tai, G.-P.; Li, Q.-S.; Gao, L.; Ruan, B.-F. Synthesis and in vitro and in vivo anti-inflammatory activity of novel 4-ferrocenylchroman-2-one derivatives. J. Enzym. Inhib. Med. Chem. 2019, 34, 1678–1689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, A.; Lumb, I.; Mehra, V.; Kumar, V. Ferrocene-appended pharmacophores: An exciting approach for modulating the biological potential of organic scaffolds. Dalton Trans. 2019, 48, 2840–2860. [Google Scholar] [CrossRef]
- Johnson, E.R.; Keinan, S.; Mori-Sánchez, P.; Contreras-García, J.; Cohen, A.J.; Yang, W. Revealing noncovalent interactions. J. Am. Chem. Soc. 2010, 132, 6498–6506. [Google Scholar] [CrossRef] [Green Version]
- Kovacic, P.; Pozos, R.S.; Somanathan, R.; Shangari, N.; O’Brien, P.J. Mechanism of mitochondrial uncouplers, inhibitors, and toxins: Focus on electron transfer, free radicals, and structure-activity relationships. Curr. Med. Chem. 2005, 12, 2601–2623. [Google Scholar] [CrossRef] [PubMed]
- González-Mojica, N.; Almazán-Sánchez, L.; García-Torres, J.G.; Santana-Martinez, I.; Martínez-Otero, D.; Sánchez-Carmona, M.A.; Cuevas-Yañez, E. Oxidation of 1, 4-disubstituted-1, 2, 3-triazoles with H2O2–CF3CO2H: Efficient synthesis of 1, 2, 3-triazole 3-oxides. Synth. Commun. 2019, 49, 679–687. [Google Scholar] [CrossRef]
- Carr, A.C.; McCall, M.R.; Frei, B. Oxidation of LDL by myeloperoxidase and reactive nitrogen species: Reaction pathways and antioxidant protection. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1716–1723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oates, J.C.; Shaftman, S.R.; Self, S.E.; Gilkeson, G.S. Association of serum nitrate and nitrite levels with longitudinal assessments of disease activity and damage in systemic lupus erythematosus and lupus nephritis. Arthritis Rheum. 2008, 58, 263–272. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Gao, M.; Li, J.; Sun, J.; Wu, R.; Han, D.; Tan, J.; Wang, J.; Wang, B.; Zhang, L. MMP-9-positive neutrophils are essential for establishing profibrotic microenvironment in the obstructed kidney of UUO mice. Acta Physiol. 2019, 227, e13317. [Google Scholar] [CrossRef]
- Perrone, M.G.; Vitale, P.; Panella, A.; Fortuna, C.G.; Scilimati, A. General role of the amino and methylsulfamoyl groups in selective cyclooxygenase (COX)-1 inhibition by 1, 4-diaryl-1, 2, 3-triazoles and validation of a predictive pharmacometric PLS model. Eur. J. Med. Chem. 2015, 94, 252–264. [Google Scholar] [CrossRef]
- Chuang, D.Y.; Simonyi, A.; Kotzbauer, P.T.; Gu, Z.; Sun, G.Y. Cytosolic phospholipase A 2 plays a crucial role in ROS/NO signaling during microglial activation through the lipoxygenase pathway. J. Neuroinflammation 2015, 12, 199. [Google Scholar] [CrossRef] [Green Version]
- Ng, C.Y.; Kannan, S.; Chen, Y.J.; Tan, F.C.K.; Ong, W.Y.; Go, M.L.; Verma, C.S.; Low, C.-M.; Lam, Y. A new generation of arachidonic acid analogues as potential neurological agent targeting cytosolic phospholipase A 2. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassan, G.S.; Abou-Seri, S.M.; Kamel, G.; Ali, M.M. Celecoxib analogs bearing benzofuran moiety as cyclooxygenase-2 inhibitors: Design, synthesis and evaluation as potential anti-inflammatory agents. Eur. J. Med. Chem. 2014, 76, 482–493. [Google Scholar] [CrossRef] [PubMed]
- Kitchen, D.B.; Decornez, H.; Furr, J.R.; Bajorath, J. Docking and scoring in virtual screening for drug discovery: Methods and applications. Nat. Rev. Drug Discov. 2004, 3, 935–949. [Google Scholar] [CrossRef] [PubMed]
- Schwede, T.; Kopp, J.; Guex, N.; Peitsch, M.C. SWISS-MODEL: An automated protein homology-modeling server. Nucleic Acids Res. 2003, 31, 3381–3385. [Google Scholar] [CrossRef] [Green Version]
- Mouchlis, V.D.; Michopoulou, V.; Constantinou-Kokotou, V.; Mavromoustakos, T.; Dennis, E.A.; Kokotos, G. Binding conformation of 2-oxoamide inhibitors to group IVA cytosolic phospholipase A2 determined by molecular docking combined with molecular dynamics. J. Chem. Inf. Model. 2012, 52, 243–254. [Google Scholar] [CrossRef] [Green Version]
- Stoe, C. X-Area (Version 1.18) and X-Red32 (Version 1.04); Stoe & Cie: Darmstadt, Germany, 2002. [Google Scholar]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Cheng, S.-C.; Wu, Y.-H.; Huang, W.-C.; Pang, J.-H.S.; Huang, T.-H.; Cheng, C.-Y. Anti-inflammatory property of quercetin through downregulation of ICAM-1 and MMP-9 in TNF-α-activated retinal pigment epithelial cells. Cytokine 2019, 116, 48–60. [Google Scholar] [CrossRef]
- Shultz, P.J.; Raij, L. Endogenously synthesized nitric oxide prevents endotoxin-induced glomerular thrombosis. J. Clin. Investig. 1992, 90, 1718–1725. [Google Scholar] [CrossRef]
- Cheng, F.; Li, W.; Zhou, Y.; Shen, J.; Wu, Z.; Liu, G.; Lee, P.W.; Tang, Y. admetSAR: A comprehensive source and free tool for assessment of chemical ADMET properties. J. Chem. Inf. Model. 2012, 52, 3099–3105. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Lou, C.; Sun, L.; Li, J.; Cai, Y.; Wang, Z.; Li, W.; Liu, G.; Tang, Y. admetSAR 2.0: Web-service for prediction and optimization of chemical ADMET properties. Bioinformatics 2019, 35, 1067–1069. [Google Scholar] [CrossRef]
- Sanner, M.F. Python: A programming language for software integration and development. J. Mol. Graph. Model. 1999, 17, 57–61. [Google Scholar] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeLano, W.L. PyMOL. In The PyMOL Molecular Graphics System; DeLano, W.L., Ed.; DeLano Scientific: San Carlos, CA, USA, 2002. [Google Scholar]









| Experimental Details | Crystal Data of X1 | Crystal Data of X5 |
|---|---|---|
| CCDC | 1537323 | 1537684 |
| Chemical formula | C18H14FeN4O2 | C18H14FeN4O2 |
| Mr | 374.18 | 374.18 |
| Crystal system, space group | Monoclinic, P21/c | Monoclinic, P21/c |
| Temperature (K) | 296 | 293 |
| a, b, c (Å) | 11.1502 (7), 5.7600 (2), 24.1625 (12) | 10.1528 (7), 13.9830 (6), 11.2383 (7) |
| α, β, γ (°) | 90.000 (4), 95.892 (4), 90.000 (4) | 90.000 (4), 100.475 (5), 90.000 (4) |
| V (Å3) | 1543.64 (14) | 1568.87 (16) |
| Z | 4 | 4 |
| Radiation type | Mo Kα | Mo Kα |
| μ (mm−1) | 1.00 | 0.98 |
| Crystal size (mm) | 0.50 × 0.25 × 0.11 | 0.72 × 0.37 × 0.13 |
| Data collection Diffractometer | STOE IPDS 2 | STOE IPDS 2 |
| Absorption correction | Integration (X-RED32; Stoe & Cie, 2002) | Integration (X-RED32; Stoe & Cie, 2002) |
| Tmin, Tmax | 0.755, 0.945 | 0.675, 0.900 |
| No. of measured, independent and observed [I > 2 σ (I)] reflections | 8127, 2976, 2356 | 7441, 3027, 2510 |
| Rint | 0.028 | 0.027 |
| (sin θ/λ) max (Å−1) | 0.614 | 0.615 |
| Refinement R [F2 > 2 σ (F2)], wR (F2), S | 0.035, 0.094, 1.01 | 0.028, 0.073, 1.04 |
| No. of reflections | 2976 | 3027 |
| No. of parameters | 226 | 226 |
| No. of restraints | 12 | 0 |
| H-atom treatment | H-atom parameters constrained | H-atom parameters constrained |
| Δρmax, Δρmin (e Å−3) | 0.58, −0.36 | 0.21, −0.22 |
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| Compound X1 | ||||
| C17—H17⋯O1i | 0.93 | 2.53 | 3.388 (3) | 154.4 |
| Compound X5 | ||||
| C14—H14⋯N2i | 0.93 | 2.57 | 3.428 (2) | 154.4 |
| Code | Physicochemical Properties | Pharmacokinetics | Toxicity Parameters | ||||||
|---|---|---|---|---|---|---|---|---|---|
| MWa | HBAb | HBDc | RBd | logP | GIAe | OBf | Carcino genesis | Irritation | |
| X1 | 374.18 | 5 | 0 | 3 | 3.96 | + | + | − | + |
| X2 | 495.28 | 7 | 0 | 5 | 5.54 | + | + | − | − |
| X3 | 381.62 | 3 | 0 | 2 | 4.85 | + | + | − | − |
| X4 | 408.08 | 3 | 0 | 2 | 4.82 | + | + | − | + |
| X5 | 374.18 | 5 | 0 | 3 | 3.96 | + | + | − | + |
| PDB | Compound Code | H-bonding Site Residues | Binding Energy (kcal/mol) | Distance (A) |
|---|---|---|---|---|
| 6COX | X1 | Arg120, Tyr355 | −9.04 | 2.9, 3.1 |
| 6COX | X2 | Gln203, Tyr409 | −10.33 | 2.7, 3.0, 3.2 |
| 6COX | X3 | Tyr115 | −7.75 | 2.9 |
| 6COX | X4 | Tyr115 | −8.23 | 2.9, 3.0 |
| 6COX | X5 | Arg513, Tyr355, Ser353, His90 | −9.35 | 2.7, 2.8, 3.1, 3.4 |
| 1CJY | X1 | Gly551, Leu552, Lys595 | −6.97 | 3.5, 3.2, 2.6 |
| 1CJY | X2 | Gly197, Gly198, Ser228, Asp549, Gly551 | −11.51 | 2.5, 2.6, 2.8, 3.0 |
| 1CJY | X3 | Asp345 | −9.13 | 2.8 |
| 1CJY | X4 | Asp345, Trp346, Pro559 | −9.94 | 2.9, 3.2, 3.4 |
| 1CJY | X5 | Asn262, Val407, Asn682, Gly684 | −7.15 | 2.7, 2.8, 3.0, 3.1, 3.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, C.-Y.; Haque, A.; Hsieh, M.-F.; Imran Hassan, S.; Faizi, M.S.H.; Dege, N.; Khan, M.S. 1,4-Disubstituted 1H-1,2,3-Triazoles for Renal Diseases: Studies of Viability, Anti-Inflammatory, and Antioxidant Activities. Int. J. Mol. Sci. 2020, 21, 3823. https://doi.org/10.3390/ijms21113823
Cheng C-Y, Haque A, Hsieh M-F, Imran Hassan S, Faizi MSH, Dege N, Khan MS. 1,4-Disubstituted 1H-1,2,3-Triazoles for Renal Diseases: Studies of Viability, Anti-Inflammatory, and Antioxidant Activities. International Journal of Molecular Sciences. 2020; 21(11):3823. https://doi.org/10.3390/ijms21113823
Chicago/Turabian StyleCheng, Ching-Yi, Ashanul Haque, Ming-Fa Hsieh, Syed Imran Hassan, Md. Serajul Haque Faizi, Necmi Dege, and Muhammad S. Khan. 2020. "1,4-Disubstituted 1H-1,2,3-Triazoles for Renal Diseases: Studies of Viability, Anti-Inflammatory, and Antioxidant Activities" International Journal of Molecular Sciences 21, no. 11: 3823. https://doi.org/10.3390/ijms21113823
APA StyleCheng, C. -Y., Haque, A., Hsieh, M. -F., Imran Hassan, S., Faizi, M. S. H., Dege, N., & Khan, M. S. (2020). 1,4-Disubstituted 1H-1,2,3-Triazoles for Renal Diseases: Studies of Viability, Anti-Inflammatory, and Antioxidant Activities. International Journal of Molecular Sciences, 21(11), 3823. https://doi.org/10.3390/ijms21113823