Testing the Effectiveness of Curcuma longa Leaf Extract on a Skin Equivalent Using a Pumpless Skin-on-a-Chip Model
Abstract
:1. Introduction
2. Results
2.1. Examination of Tissue Morphology with H&E Staining
2.2. Measurement of the Thickness of the Stratum Corneum
2.3. qRT-PCR Results for Quantitative Analysis
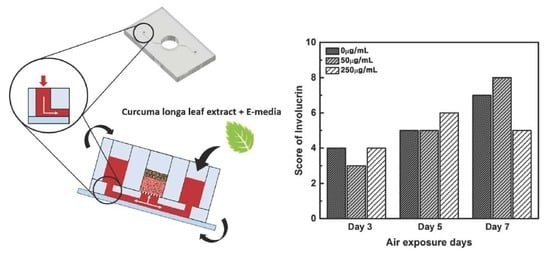
2.4. Immunohistochemistry (IHC) Result of Staining Analysis
3. Discussion
4. Materials and Methods
4.1. Fabrication of a Pumpless Microfluidic Chip and Gravity Flow System
4.2. Construction of a 3D Skin Equivalent Model
4.3. Real-time Quantitative Analysis
4.4. H&E Staining and Immunohistochemistry
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CLLE | Curcuma longa Leaf extract |
| RTC | Rat Tail Collagen |
| ECM | Extra-Cellular Medium |
| PDMS | Polydimethylsiloxane |
| IHC | Immunohistochemistry |
References
- Monks, A.; Scudiero, D.; Skehan, P.; Shoemaker, R.; Paull, K.; Vistica, D.; Hose, C.; Langley, J.; Cronise, P.; Vaigro-Wolff, A.; et al. Feasibility of a High-Flux Anticancer Drug Screen Using a Diverse Panel of Cultured Human Tumor Cell Lines. JNCI J. Natl. Cancer Inst. 1991, 83, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Alley, M.C.; Scudiero, D.A.; Monks, A.; Hursey, M.; Czerwinski, M.J.; Fine, D.L.; Abbott, B.J.; Mayo, J.G.; Shoemaker, R.; Boyd, M.R. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res. 1988, 48, 584–588. [Google Scholar]
- Song, H.J.; Lim, H.Y.; Chun, W.; Choi, K.C.; Sung, J.H.; Sung, G.Y. Fabrication of a pumpless, microfluidic skin chip from different collagen sources. J. Ind. Eng. Chem. 2017, 56, 375–381. [Google Scholar] [CrossRef]
- Song, H.J.; Lim, H.Y.; Chun, W.; Choi, K.C.; Lee, T.; Sung, J.H.; Sung, G.Y. Development of 3D skin-equivalent in a pump-less microfluidic chip. J. Ind. Eng. Chem. 2018, 60, 355–359. [Google Scholar] [CrossRef]
- Lim, H.Y.; Kim, J.; Song, H.J.; Kim, K.; Choi, K.C.; Park, S.; Sung, G.Y. Development of wrinkled skin-on-a-chip (WSOC) by cyclic uniaxial stretching. J. Ind. Eng. Chem. 2018, 68, 238–245. [Google Scholar] [CrossRef]
- Lilienblum, W.; Dekant, W.; Foth, H.; Gebel, T.; Hengstler, J.G.; Kahl, R.; Kramer, P.J.; Schweinfurth, H.; Wollin, K.M. Alternative methods to safety studies in experimental animals: Role in the risk assessment of chemicals under the new European Chemicals Legislation (REACH). Arch. Toxicol. 2008, 82, 211–236. [Google Scholar] [CrossRef]
- Almeida, A.; Sarmento, B.; Rodrigues, F. Insights on in vitro models for safety and toxicity assessment of cosmetic ingredients. Int. J. Pharm. 2017, 519, 178–185. [Google Scholar] [CrossRef]
- Maeno, S.; Niki, Y.; Matsumoto, H.; Morioka, H.; Yatabe, T.; Funayama, A.; Toyama, Y.; Taguchi, T.; Tanaka, J. The effect of calcium ion concentration on osteoblast viability, proliferation and differentiation in monolayer and 3D culture. Biomaterials 2005, 26, 4847–4855. [Google Scholar] [CrossRef]
- Lovitt, C.; Shelper, T.; Avery, V.; Lovitt, C.J.; Shelper, T.B.; Avery, V.M. Advanced Cell Culture Techniques for Cancer Drug Discovery. Biology 2014, 3, 345–367. [Google Scholar] [CrossRef] [Green Version]
- Dhiman, H.K.; Ray, A.R.; Panda, A.K. Three-dimensional chitosan scaffold-based MCF-7 cell culture for the determination of the cytotoxicity of tamoxifen. Biomaterials 2005, 26, 979–986. [Google Scholar] [CrossRef]
- Ihle, N.T.; Williams, R.; Chow, S.; Chew, W.; Berggren, M.I.; Paine-Murrieta, G.; Minion, D.J.; Halter, R.J.; Wipf, P.; Abraham, R.; et al. Molecular pharmacology and antitumor activity of PX-866, a novel inhibitor of phosphoinositide-3-kinase signaling. Mol. Cancer Ther. 2004, 3, 763–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandilands, A.; Sutherland, C.; Irvine, A.D.; McLean, W.H.I. Filaggrin in the frontline: Role in skin barrier function and disease. J. Cell Sci. 2009, 122, 1285–1294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Labban, L. Medicinal and pharmacological properties of Turmeric (Curcuma longa): A review. Int. J. Pharm. Biomed. Res. 2014, 5, 17–23. [Google Scholar]
- Jayaprakasha, G.K.; Jagan Mohan Rao, L.; Sakariah, K.K. Chemistry and biological activities of C. longa. Trends Food Sci. Technol. 2005, 16, 533–548. [Google Scholar] [CrossRef]
- Sandur, S.K.; Pandey, M.K.; Sung, B.; Ahn, K.S.; Murakami, A.; Sethi, G.; Limtrakul, P.; Badmaev, V.; Aggarwal, B.B. Curcumin, demethoxycurcumin, bisdemethoxycurcumin, tetrahydrocurcumin and turmerones differentially regulate anti-inflammatory and anti-proliferative responses through a ROS-independent mechanism. Carcinogenesis 2007, 28, 1765–1773. [Google Scholar] [CrossRef] [PubMed]
- He, X.-G.; Lin, L.-Z.; Lian, L.-Z.; Lindenmaier, M. Liquid chromatography–electrospray mass spectrometric analysis of curcuminoids and sesquiterpenoids in turmeric (Curcuma longa). J. Chromatogr. A 1998, 818, 127–132. [Google Scholar] [CrossRef]
- Kim, N.Y.; Lee, H.Y. Enhancement of Skin Immune Activities of Curcuma longa L. Leaf Extract by Ultra High Pressure Process. Korean J. Med. Crop Sci. 2014, 22, 378–383. [Google Scholar] [CrossRef] [Green Version]
- Kim, N.Y.; Lim, H.W.; Lee, H.Y. Comparison of Cosmetical Activities of Curcuma longa L. Leaf Extracts Using Different Extraction Methods. Korean J. Med. Crop Sci. 2014, 22, 255–261. [Google Scholar] [CrossRef] [Green Version]
- Choi, W.Y.; Lee, H.Y. Enhancement of Antioxidant Activities of Curcuma longa Leaves by Ultra High Pressure Extraction. Korean J. Med. Crop Sci. 2014, 22, 121–126. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.-E.; Kwon, S.; Wu, G.; Kim, D.; Park, B.K.; Park, J.-A.; Choi, K.-C.; Kim, D.-S.; Kwon, H.-J.; Lee, Y. Therapeutic effect of a TM4SF5-specific monoclonal antibody against colon cancer in a mouse model. Oncotarget 2014, 5, 8402–8415. [Google Scholar] [CrossRef] [Green Version]
- Netzlaff, F.; Lehr, C.-M.; Wertz, P.W.; Schaefer, U.F. The human epidermis models EpiSkin®, SkinEthic® and EpiDerm®: An evaluation of morphology and their suitability for testing phototoxicity, irritancy, corrosivity, and substance transport. Eur. J. Pharm. Biopharm. 2005, 60, 167–178. [Google Scholar] [CrossRef]
- Ponec, M.; Boelsma, E.; Weerheim, A.; Mulder, A.; Bouwstra, J.; Mommaas, M. Lipid and ultrastructural characterization of reconstructed skin models. Int. J. Pharm. 2000, 203, 211–225. [Google Scholar] [CrossRef]
- Madison, K.C. Barrier Function of the Skin: “La Raison d’Être” of the Epidermis. J. Investig. Dermatol. 2003, 121, 231–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuchs, E.; Esteves, R.A.; Coulombe, P.A. Transgenic mice expressing a mutant keratin 10 gene reveal the likely genetic basis for epidermolytic hyperkeratosis. Proc. Natl. Acad. Sci. USA 1992, 89, 6906–6910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenthal, D.S.; Griffiths, C.E.M.; Yuspa, S.H.; Roop, D.R.; Voorhees, J.J. Acute or Chronic Topical Retinoic Acid Treatment of Human Skin In Vivo Alters the Expression of Epidermal Transglutaminase, Loricrin, Involucrin, Filaggrin, and Keratins 6 and 13 but not Keratins 1, 10, and 14. J. Investig. Dermatol. 1992, 98, 343–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frank, D.E.; Carter, W.G. Laminin 5 deposition regulates keratinocyte polarization and persistent migration. J. Cell Sci. 2004, 117, 1351–1363. [Google Scholar] [CrossRef] [Green Version]
- O’Toole, E.A.; Marinkovich, M.P.; Hoeffler, W.K.; Furthmayr, H.; Woodley, D.T. Laminin-5 Inhibits Human Keratinocyte Migration. Exp. Cell Res. 1997, 233, 330–339. [Google Scholar] [CrossRef]
- Aumailley, M.; Smyth, N. The role of laminins in basement membrane function. J. Anat. 1998, 193 Pt 1, 1–21. [Google Scholar] [CrossRef]
- Fyrand, O. Studies on fibronectin in the skin. Arch. Dermatol. Res. 1979, 266, 33–41. [Google Scholar] [CrossRef]
- Clark, R.A.F. Fibronectin Matrix Deposition and Fibronectin Receptor Expression in Healing and Normal Skin. J. Investig. Dermatol. 1990, 94, s128–s134. [Google Scholar] [CrossRef] [Green Version]
- Fleischmajer, R.; Dessau, W.; Timpl, R.; Krieg, T.; Luderschmidt, C.; Wiestner, M. Immunofluorescence Analysis of Collagen, Fibronectin, and Basement Membrane Protein in Scleroderma Skin. J. Investig. Dermatol. 1980, 75, 270–274. [Google Scholar] [CrossRef] [PubMed]
- McGrath, J.A.; Uitto, J. The filaggrin story: Novel insights into skin-barrier function and disease. Trends Mol. Med. 2008, 14, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Freshney, R.I. Culture of Specific Cell Types. In Culture of Animal Cells; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005. [Google Scholar]
- Said, J.W.; Sassoon, A.F.; Shintaku, I.P.; Banks-Schlegel, S. Involucrin in Squamous and Basal cell Carcinomas of the Skin: An Immunohistochemical Study. J. Investig. Dermatol. 1984, 82, 449–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, G.F.; Flynn, T.C.; Rice, R.H.; Pinkus, G.S. Involucrin Expression in Normal and Neoplastic Human Skin: A Marker for Keratinocyte Differentiation. J. Investig. Dermatol. 1984, 82, 453–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Packer, L.; Wltr, E.H.; Tritschler, H.J. Alpha-lipoic acid as a biological antioxidant. Free Radic. Biol. Med. 1995, 19, 227–250. [Google Scholar] [CrossRef]
- Eckert, R.L.; Crish, J.F.; Efimova, T.; Dashti, S.R.; Deucher, A.; Bone, F.; Adhikary, G.; Huang, G.; Gopalakrishnan, R.; Balasubramanian, S. Regulation of Involucrin Gene Expression. J. Investig. Dermatol. 2004, 123, 13–22. [Google Scholar] [CrossRef] [Green Version]
- Kalinin, A.E.; Kajava, A.V.; Steinert, P.M. Epithelial barrier function: Assembly and structural features of the cornified cell envelope. BioEssays 2002, 24, 789–800. [Google Scholar] [CrossRef]
- Lane, E.; McLean, W. Keratins and skin disorders. J. Pathol. 2004, 204, 355–366. [Google Scholar] [CrossRef]
- Proksch, E.; Fölster-Holst, R.; Jensen, J.-M. Skin barrier function, epidermal proliferation and differentiation in eczema. J. Dermatol. Sci. 2006, 43, 159–169. [Google Scholar] [CrossRef]
- Santos, M.; Paramio, J.M.; Bravo, A.; Ramirez, A.; Jorcano, J.L. The expression of keratin k10 in the basal layer of the epidermis inhibits cell proliferation and prevents skin tumorigenesis. J. Biol. Chem. 2002, 277, 19122–19130. [Google Scholar] [CrossRef] [Green Version]
- Mommers, J.M.; van Rossum, M.M.; van Erp, P.E.J.; van de Kerkhof, P.C.M. Changes in Keratin 6 and Keratin 10 (Co-)Expression in Lesional and Symptomless Skin of Spreading Psoriasis. Dermatology 2000, 201, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.-M.; Schütze, S.; Neumann, C.; Proksch, E. Impaired Cutaneous Permeability Barrier Function, Skin Hydration, and Sphingomyelinase Activity in Keratin 10 Deficient Mice. J. Investig. Dermatol. 2000, 115, 708–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]










© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.; Jeon, H.M.; Choi, K.C.; Sung, G.Y. Testing the Effectiveness of Curcuma longa Leaf Extract on a Skin Equivalent Using a Pumpless Skin-on-a-Chip Model. Int. J. Mol. Sci. 2020, 21, 3898. https://doi.org/10.3390/ijms21113898
Kim K, Jeon HM, Choi KC, Sung GY. Testing the Effectiveness of Curcuma longa Leaf Extract on a Skin Equivalent Using a Pumpless Skin-on-a-Chip Model. International Journal of Molecular Sciences. 2020; 21(11):3898. https://doi.org/10.3390/ijms21113898
Chicago/Turabian StyleKim, Kyunghee, Hye Mi Jeon, Kyung Chan Choi, and Gun Yong Sung. 2020. "Testing the Effectiveness of Curcuma longa Leaf Extract on a Skin Equivalent Using a Pumpless Skin-on-a-Chip Model" International Journal of Molecular Sciences 21, no. 11: 3898. https://doi.org/10.3390/ijms21113898
APA StyleKim, K., Jeon, H. M., Choi, K. C., & Sung, G. Y. (2020). Testing the Effectiveness of Curcuma longa Leaf Extract on a Skin Equivalent Using a Pumpless Skin-on-a-Chip Model. International Journal of Molecular Sciences, 21(11), 3898. https://doi.org/10.3390/ijms21113898