Crosstalk between Hydrogen Sulfide and Other Signal Molecules Regulates Plant Growth and Development
Abstract
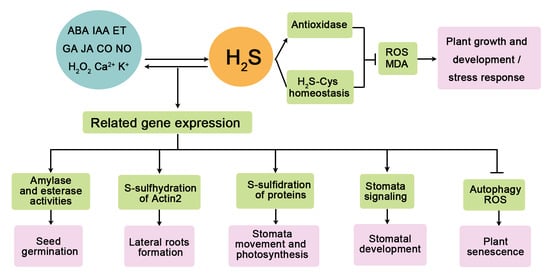
:1. Introduction
2. Roles of H2S at Different Stages of Plant Development
2.1. H2S Promotes Seed Germination
2.2. H2S Affects Formation of Lateral Roots
2.3. H2S Regulates Plant Stomata Movement and Photosynthesis
2.4. H2S Delays Plant Senescence
2.4.1. H2S Delays Leaf Senescence
2.4.2. H2S Delays the Postharvest Maturation of Fruits
2.4.3. H2S Inhibits Organ Abscission in Plants
3. Mechanism by which H2S Regulates Plant Growth and Development
3.1. Crosstalk of H2S with Plant Hormones
3.1.1. Crosstalk of H2S with Abscisic Acid
3.1.2. Crosstalk of H2S with Ethylene
3.1.3. Crosstalk of H2S with Auxin
3.1.4. Crosstalk of H2S with Gibberellin
3.1.5. Crosstalk of H2S with Salicylic Acid
3.1.6. Crosstalk of H2S with Jasmonate
3.2. Crosstalk between H2S and Other Gasotransmitters
3.2.1. Crosstalk between H2S and NO
3.2.2. Crosstalk between H2S and CO
3.3. Crosstalk of H2S with Ionic Signals
3.3.1. Crosstalk of H2S with Ca2+
3.3.2. Crosstalk of H2S with Na+ and K+
3.4. S-sulfhydration Modification of Proteins Mediated by H2S
4. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| S | Sulfur |
| Cys | Cysteine |
| L-Cys | L-cysteine |
| Met | Methionine |
| H2S | Hydrogen sulfide |
| SIR | Sulfite reductase |
| OAS-TL | O-acetylserine (thiol) lyase |
| OAS | O-acetylserine |
| APS | Adenosine 5′-phosphosulfate |
| ATPS | ATP sulfurylase |
| APSR | Adenosine- 5′- phosphoryl sulfate reductase |
| CDes | Cysteine desulfhydrase |
| LCD | L-cysteine desulfhydrase |
| DES1 | L-cysteine desulfhydrase 1 |
| DCD1 | D-cysteine desulfhydrase1 |
| DCD2 | D-cysteine desulfhydrase2 |
| CAS | β-cyanoalanine synthase |
| CN− | Cyanide |
| GSH | Glutathione |
| AOA | Aminooxyacetic acid, an H2S synthesis inhibitor |
| HT | Hypotaurine, an H2S scavenger |
| PAG | Propargylglycine, a DES1 inhibitor |
| NPA | N-1-naphthylphthalamic acid, IAA transport inhibitor |
| Cptio | 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide, NO scavenger |
| GSNOR1 | S-nitrosoglutathione reductase 1 |
| GAPDH | Glyceraldehyde phosphate dehydrogenase |
| SNP | Sodium nitroprusside, NO donor |
| HA | Hydroxylamine, H2S synthesis inhibitor |
References
- Takahashi, H.; Kopriva, S.; Giordano, M.; Saito, K.; Hell, R. Sulfur assimilation in photosynthetic organisms: Molecular functions and regulations of transporters and assimilatory enzymes. Annu. Rev. Plant Biol. 2011, 62, 157–184. [Google Scholar] [CrossRef] [PubMed]
- Rennenberg, H.; Arabatzis, N.; Grundel, I.J.P. Cysteine desulphydrase activity in higher plants: Evidence for the action of L- and D-cysteine specific enzymes. Phytochemistry 1987, 26, 1583–1589. [Google Scholar] [CrossRef]
- Li, Z.G. Analysis of some enzymes activities of hydrogen sulfide metabolism in plants. Method Enzymol. 2015, 555, 253–269. [Google Scholar]
- Romero, L.C.; Garcia, I.; Gotor, C. L-Cysteine Desulfhydrase 1 modulates the generation of the signaling molecule sulfide in plant cytosol. Plant Signal. Behav. 2013, 8, e24007. [Google Scholar] [CrossRef] [Green Version]
- Leon, S.; Touraine, B.; Briat, J.F.; Lobreaux, S. The AtNFS2 gene from Arabidopsis thaliana encodes a NifS-like plastidial cysteine desulphurase. Biochem. J. 2002, 366, 557–564. [Google Scholar] [CrossRef]
- Hatzfeld, Y.; Maruyama, A.; Schmidt, A.; Noji, M.; Ishizawa, K.; Saito, K. beta-cyanoalanine synthase is a mitochondrial cysteine synthase-like protein in spinach and Arabidopsis. Plant Physiol. 2000, 123, 1163–1171. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, Y.; Nakamura, T.; Kusano, T.; Sano, H. Three Arabidopsis genes encoding proteins with differential activities for cysteine synthase and beta-cyanoalanine synthase. Plant Cell Physiol. 2000, 41, 465–476. [Google Scholar] [CrossRef]
- Hancock, J.T. Harnessing evolutionary toxins for signaling: Reactive oxygen species, nitric oxide and hydrogen sulfide in plant cell regulation. Front. Plant Sci. 2017, 8, 189. [Google Scholar] [CrossRef] [Green Version]
- Mancardi, D.; Penna, C.; Merlino, A.; Del Soldato, P.; Wink, D.A.; Pagliaro, P. Physiological and pharmacological features of the novel gasotransmitter: Hydrogen sulfide. BBA Bioenergetics 2009, 1787, 864–872. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.G.; Min, X.; Zhou, Z.H. Hydrogen sulfide: A signal molecule in plant cross-adaptation. Front. Plant Sci. 2016, 7, 1621. [Google Scholar] [CrossRef] [Green Version]
- Baudouin, E.; Poilevey, A.; Hewage, N.I.; Cochet, F.; Puyaubert, J.; Bailly, C. The significance of hydrogen sulfide for Arabidopsis seed germination. Front. Plant Sci. 2016, 7, 930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Hu, L.Y.; Hu, K.D.; He, Y.D.; Wang, S.H.; Luo, J.P. Hydrogen sulfide promotes wheat seed germination and alleviates oxidative damage against copper stress. J. Integr. Plant Biol. 2008, 50, 1518–1529. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.P.; Pei, Y.X. Hydrogen sulfide: The shutter button of stomata in plants. Sci. China Life Sci. 2016, 59, 1187–1188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lisjak, M.; Srivastava, N.; Teklic, T.; Civale, L.; Lewandowski, K.; Wilson, I.; Wood, M.E.; Whiteman, M.; Hancock, J.T. A novel hydrogen sulfide donor causes stomatal opening and reduces nitric oxide accumulation. Plant Physiol. Biochem. 2010, 48, 931–935. [Google Scholar] [CrossRef]
- Alvarez, C.; Garcia, I.; Moreno, I.; Perez-Perez, M.E.; Crespo, J.L.; Romero, L.C.; Gotor, C. Cysteine-generated sulfide in the cytosol negatively regulates autophagy and modulates the transcriptional profile in Arabidopsis. Plant Cell 2012, 24, 4621–4634. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Wu, F.H.; Wang, W.H.; Zheng, C.J.; Lin, G.H.; Dong, X.J.; He, J.X.; Pei, Z.M.; Zheng, H.L. Hydrogen sulphide enhances photosynthesis through promoting chloroplast biogenesis, photosynthetic enzyme expression, and thiol redox modification in Spinacia oleracea seedlings. J. Exp. Bot. 2011, 62, 4481–4493. [Google Scholar] [CrossRef] [Green Version]
- Ali, S.; Nawaz, A.; Ejaz, S.; Haider, S.T.A.; Alam, M.W.; Javed, H.U. Effects of hydrogen sulfide on postharvest physiology of fruits and vegetables: An overview. Sci. Hortic. 2019, 243, 290–299. [Google Scholar] [CrossRef]
- Liu, D.M.; Li, J.N.; Li, Z.W.; Pei, Y.X. Hydrogen sulfide inhibits ethylene-induced petiole abscission in tomato (Solanum lycopersicum L.). Hortic. Res. England 2020, 7, 14. [Google Scholar] [CrossRef] [Green Version]
- Li, J.S.; Chen, S.S.; Wang, X.F.; Shi, C.; Liu, H.X.; Yang, J.; Shi, W.; Guo, J.K.; Jia, H.L. Hydrogen sulfide disturbs actin polymerization via S-sulfhydration resulting in stunted root hair growth. Plant Physiol. 2018, 178, 936–949. [Google Scholar] [CrossRef] [Green Version]
- Yuan, X.; Wen, B. Seed germination response to high temperature and water stress in three invasive Asteraceae weeds from Xishuangbanna, SW China. PLoS ONE 2018, 13, e0191710. [Google Scholar] [CrossRef] [Green Version]
- Dooley, F.D.; Nair, S.P.; Ward, P.D. Increased growth and germination success in plants following hydrogen sulfide administration. PLoS ONE 2013, 8, e62048. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Wang, M.J.; Hu, L.Y.; Wang, S.H.; Hu, K.D.; Bao, L.J.; Luo, J.P. Hydrogen sulfide promotes wheat seed germination under osmotic stress. Russ. J. Plant Physl. 2010, 57, 532–539. [Google Scholar] [CrossRef]
- Zhang, H.; Tan, Z.Q.; Hu, L.Y.; Wang, S.H.; Luo, J.P.; Jones, R.L. Hydrogen sulfide alleviates aluminum toxicity in germinating wheat seedlings. J. Integr. Plant Biol. 2010, 52, 556–567. [Google Scholar] [CrossRef]
- Chen, Z.; Huang, Y.W.; Yang, W.J.; Chang, G.X.; Li, P.; Wei, J.L.; Yuan, X.J.; Huang, J.L.; Hu, X.Y. The hydrogen sulfide signal enhances seed germination tolerance to high temperatures by retaining nuclear COP1 for HY5 degradation. Plant Sci. 2019, 285, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.H.; Wang, Y.; Ye, X.Y.; Li, Z.G. Signaling molecule hydrogen sulfide improves seed germination and seedling growth of maize (Zea mays L.) under high temperature by inducing antioxidant system and osmolyte biosynthesis. Front. Plant Sci. 2018, 9, 1288. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.G.; Gong, M.; Liu, P. Hydrogen sulfide is a mediator in H2O2-induced seed germination in Jatropha Curcas. Acta Physiol. Plant 2012, 34, 2207–2213. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Y.; Li, X.; Xue, R.L.; Zhao, H.J. Identification of wheat d-cysteine desulfhydrase (TaD-CDes) required for abscisic acid regulation of seed germination, root growth, and stomatal closure in Arabidopsis. J. Plant Growth Regul. 2018, 37, 1175–1184. [Google Scholar] [CrossRef]
- Casimiro, I.; Marchant, A.; Bhalerao, R.P.; Beeckman, T.; Dhooge, S.; Swarup, R.; Graham, N.; Inze, D.; Sandberg, G.; Casero, P.J.; et al. Auxin transport promotes Arabidopsis lateral root initiation. Plant Cell 2001, 13, 843–852. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Tang, J.; Liu, X.P.; Wang, Y.; Yu, W.; Peng, W.Y.; Fang, F.; Ma, D.F.; Wei, Z.J.; Hu, L.Y. Hydrogen sulfide promotes root organogenesis in Ipomoea batatas, Salix matsudana and Glycine max. J. Integr. Plant Biol. 2009, 51, 1086–1094. [Google Scholar] [CrossRef]
- Chen, Y.; Mo, H.Z.; Zheng, M.Y.; Xian, M.; Qi, Z.Q.; Li, Y.Q.; Hu, L.B.; Chen, J.; Yang, L.F. Selenium inhibits root elongation by repressing the generation of endogenous hydrogen sulfide in Brassica rapa. PLoS ONE 2014, 9, e110904. [Google Scholar] [CrossRef]
- Mei, Y.D.; Chen, H.T.; Shen, W.B.; Shen, W.; Huang, L.Q. Hydrogen peroxide is involved in hydrogen sulfide-induced lateral root formation in tomato seedlings. BMC Plant Biol. 2017, 17, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Fang, T.; Cao, Z.Y.; Li, J.L.; Shen, W.B.; Huang, L.Q. Auxin-induced hydrogen sulfide generation is involved in lateral root formation in tomato. Plant Physiol. Biochem. 2014, 76, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.; Chen, Z.P.; Cui, W.T.; Zhang, Y.H.; Hu, H.L.; Yu, X.L.; Wang, Q.Y.; Shen, W.B. Methane alleviates alfalfa cadmium toxicity via decreasing cadmium accumulation and reestablishing glutathione homeostasis. Ecotoxicol. Environ. Saf. 2018, 147, 861–871. [Google Scholar] [CrossRef]
- Zhu, K.K.; Cui, W.T.; Dai, C.; Wu, M.Z.; Zhang, J.; Zhang, Y.H.; Xie, Y.J.; Shen, W.B. Methane-rich water alleviates NaCl toxicity during alfalfa seed germination. Environ. Exp. Bot. 2016, 129, 37–47. [Google Scholar] [CrossRef]
- Han, B.; Duan, X.L.; Wang, Y.; Zhu, K.K.; Zhang, J.; Wang, R.; Hu, H.L.; Qi, F.; Pan, J.C.; Yan, Y.X.; et al. Methane protects against polyethylene glycol-induced osmotic stress in maize by improving sugar and ascorbic acid metabolism. Sci. Rep. UK 2017, 7, 7–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, F.; Xiang, Z.X.; Kou, N.H.; Cui, W.T.; Xu, D.K.; Wang, R.; Zhu, D.; Shen, W.B. Nitric oxide is involved in methane-induced adventitious root formation in cucumber. Physiol. Plantarum. 2017, 159, 366–377. [Google Scholar] [CrossRef] [Green Version]
- Kou, N.H.; Xiang, Z.X.; Cui, W.T.; Li, L.N.; Shen, W.B. Hydrogen sulfide acts downstream of methane to induce cucumber adventitious root development. J. Plant Physiol. 2018, 228, 113–120. [Google Scholar] [CrossRef]
- Mei, Y.D.; Zhao, Y.Y.; Jin, X.X.; Wang, R.; Xu, N.; Hu, J.W.; Huang, L.Q.; Guan, R.Z.; Shen, W.B. L-Cysteine desulfhydrase-dependent hydrogen sulfide is required for methane-induced lateral root formation. Plant Mol. Biol. 2019, 99, 283–298. [Google Scholar] [CrossRef]
- Jia, H.; Hu, Y.; Fan, T.; Li, J. Hydrogen sulfide modulates actin-dependent auxin transport via regulating ABPs results in changing of root development in Arabidopsis. Sci. Rep. UK 2015, 5, 8251. [Google Scholar] [CrossRef] [Green Version]
- Nunes, T.D.G.; Zhang, D.; Raissig, M.T. Form, development and function of grass stomata. Plant J. 2020, 101, 780–799. [Google Scholar] [CrossRef]
- Schachtman, D.P.; Goodger, J.Q.D. Chemical root to shoot signaling under drought. Trends Plant Sci. 2008, 13, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A.; Munemasa, S.; Uraji, M.; Nakamura, Y.; Mori, I.C.; Murata, Y. Involvement of endogenous abscisic acid in methyl jasmonate-induced stomatal closure in Arabidopsis. Plant Physiol. 2011, 156, 430–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Y.J.; Mao, Y.; Zhang, W.; Lai, D.W.; Wang, Q.Y.; Shen, W.B. Reactive oxygen species-dependent nitric oxide production contributes to hydrogen-promoted stomatal closure in Arabidopsis. Plant Physiol. 2014, 165, 759–773. [Google Scholar] [CrossRef] [Green Version]
- Wilkinson, S.; Davies, W.J. Ozone suppresses soil drying- and abscisic acid (ABA)-induced stomatal closure via an ethylene-dependent mechanism. Plant Cell Environ. 2009, 32, 949–959. [Google Scholar] [CrossRef]
- Hoque, T.S.; Uraji, M.; Ye, W.X.; Hossain, M.A.; Nakamura, Y.; Murata, Y. Methylglyoxal-induced stomatal closure accompanied by peroxidase-mediated ROS production in Arabidopsis. J. Plant Physiol. 2012, 169, 979–986. [Google Scholar] [CrossRef]
- Garcia-Mata, C.; Lamattina, L. Hydrogen sulphide, a novel gasotransmitter involved in guard cell signalling. New Phytol. 2010, 188, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Duan, B.B.; Ma, Y.H.; Jiang, M.R.; Yang, F.; Ni, L.; Lu, W. Improvement of photosynthesis in rice (Oryza sativa L.) as a result of an increase in stomatal aperture and density by exogenous hydrogen sulfide treatment. Plant Growth Regul. 2015, 75, 33–44. [Google Scholar] [CrossRef]
- Liu, Y.H.; Zhang, X.H.; Liu, B.W.; Ao, B.; Liu, Q.; Wen, S.Y.; Xu, Y.F. Hydrogen sulfide regulates photosynthesis of tall fescue under low-light stress. Photosynthetica 2019, 57, 714–723. [Google Scholar] [CrossRef] [Green Version]
- Tang, X.D.; An, B.Y.; Cao, D.M.; Xu, R.; Wang, S.Y.; Zhang, Z.D.; Liu, X.J.; Sun, X.G. Improving photosynthetic capacity, alleviating photosynthetic inhibition and oxidative stress under low temperature stress with exogenous hydrogen sulfide in blueberry seedlings. Front. Plant Sci. 2020, 11, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, H.L.; Chen, S.S.; Liu, D.; Liesche, J.; Shi, C.; Wang, J.; Ren, M.J.; Wang, X.F.; Yang, J.; Shi, W.; et al. Ethylene-induced hydrogen sulfide negatively regulates ethylene biosynthesis by persulfidation of ACO in tomato under osmotic stress. Front. Plant Sci. 2018, 9, 1517. [Google Scholar] [CrossRef] [Green Version]
- Hou, Z.H.; Wang, L.X.; Liu, J.; Hou, L.X.; Liu, X. Hydrogen sulfide regulates ethylene-induced stomatal closure in Arabidopsis thaliana. J. Integr. Plant Biol. 2013, 55, 277–289. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Hou, L.X.; Liu, G.H.; Liu, X.; Wang, X.C. Hydrogen sulfide induced by nitric oxide mediates ethylene-induced stomatal closure of Arabidopsis thaliana. Chin. Sci. Bull. 2011, 56, 3547–3553. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Hou, Z.H.; Liu, G.H.; Hou, L.X.; Liu, X. Hydrogen sulfide may function downstream of nitric oxide in ethylene-induced stomatal closure in Vicia faba L. J. Integr. Agric. 2012, 11, 1644–1653. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, M.J.; Ge, Z.L.; Shen, J.; Zhou, C.; Gotor, C.; Romero, L.C.; Duan, X.L.; Liu, X.; Wu, D.L.; et al. Abscisic acid-triggered guard cell l-cysteine desulfhydrase function and in situ hydrogen sulfide production contributes to heme oxygenase-modulated stomatal closure. Plant Cell Environ. 2020, 43, 624–636. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zhang, J.; Zhou, M.J.; Zhou, H.; Cui, B.M.; Gotor, C.; Romero, L.C.; Fu, L.; Yang, J.; Foyer, C.H.; et al. Persulfidation-based modification of cysteine desulfhydrase and the NADPH oxidase RBOHD controls guard cell abscisic acid signaling. Plant Cell 2020, 32, 1000–1017. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.S.; Jia, H.L.; Wang, X.F.; Shi, C.; Wang, X.; Ma, P.Y.; Wang, J.; Ren, M.J.; Li, J.S. Hydrogen sulfide positively regulates abscisic acid signaling through persulfidation of SnRK2.6 in guard cells. Mol. Plant 2020, 13, 732–744. [Google Scholar] [CrossRef] [PubMed]
- Lisjak, M.; Teklic, T.; Wilson, I.D.; Wood, M.; Whiteman, M.; Hancock, J.T. Hydrogen sulfide effects on stomatal apertures. Plant Signal. Behav. 2011, 6, 1444–1446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gregersen, P.L.; Culetic, A.; Boschian, L.; Krupinska, K. Plant senescence and crop productivity. Plant Mol. Biol. 2013, 82, 603–622. [Google Scholar] [CrossRef]
- Woo, H.R.; Masclaux-Daubresse, C.; Lim, P.O. Plant senescence: How plants know when and how to die. J. Exp. Bot. 2018, 69, 715–718. [Google Scholar] [CrossRef]
- Watanabe, M.; Tohge, T.; Balazadeh, S.; Erban, A.; Giavalisco, P.; Kopka, J.; Mueller-Roeber, B.; Fernie, A.R.; Hoefgen, R. Comprehensive metabolomics studies of plant developmental senescence. Methods Mol. Biol. 2018, 1744, 339–358. [Google Scholar]
- Zhang, H.; Hu, S.L.; Zhang, Z.J.; Hu, L.Y.; Jiang, C.X.; Wei, Z.J.; Liu, J.A.; Wang, H.L.; Jiang, S.T. Hydrogen sulfide acts as a regulator of flower senescence in plants. Postharvest Biol. Technol. 2011, 60, 251–257. [Google Scholar] [CrossRef]
- Wei, B.; Zhang, W.; Chao, J.; Zhang, T.R.; Zhao, T.T.; Noctor, G.; Liu, Y.S.; Han, Y. Functional analysis of the role of hydrogen sulfide in the regulation of dark-induced leaf senescence in Arabidopsis. Sci. Rep. UK 2017, 7, 2615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gotor, C.; Garcia, I.; Crespo, J.L.; Romero, L.C. Sulfide as a signaling molecule in autophagy. Autophagy 2013, 9, 609–611. [Google Scholar] [CrossRef] [Green Version]
- Laureano-Marin, A.M.; Moreno, I.; Romero, L.C.; Gotor, C. Negative regulation of autophagy by sulfide is independent of reactive oxygen species. Plant Physiol. 2016, 171, 1378–1391. [Google Scholar] [PubMed] [Green Version]
- Aroca, A.; Benito, J.M.; Gotor, C.; Romero, L.C. Persulfidation proteome reveals the regulation of protein function by hydrogen sulfide in diverse biological processes in Arabidopsis. J. Exp. Bot. 2017, 68, 4915–4927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filomeni, G.; Desideri, E.; Cardaci, S.; Rotilio, G.; Ciriolo, M.R. Under the ROS: Thiol network is the principal suspect for autophagy commitment. Autophagy 2010, 6, 999–1005. [Google Scholar] [CrossRef] [Green Version]
- Huo, J.Q.; Huang, D.J.; Zhang, J.; Fang, H.; Wang, B.; Wang, C.L.; Liao, W.B. Hydrogen sulfide: A gaseous molecule in postharvest freshness. Front. Plant Sci. 2018, 9, 1172. [Google Scholar] [CrossRef]
- Zhu, L.Q.; Wang, W.; Shi, J.Y.; Zhang, W.; Shen, Y.G.; Du, H.Y.; Wu, S.F. Hydrogen sulfide extends the postharvest life and enhances antioxidant activity of kiwifruit during storage. J. Sci. Food Agric. 2014, 94, 2699–2704. [Google Scholar] [CrossRef]
- Lin, X.C.; Yang, R.; Dou, Y.; Zhang, W.; Du, H.Y.; Zhu, L.Q.; Chen, J.Y. Transcriptome analysis reveals delaying of the ripening and cell-wall degradation of kiwifruit by hydrogen sulfide. J. Sci. Food Agric. 2020, 100, 2280–2287. [Google Scholar] [CrossRef]
- Gai-Fang, Y.; Zeng-Zheng, W.; Ting-Ting, L.; Jun, T.; Zhong-Qin, H.; Feng, Y.; Yan-Hong, L.; Zhuo, H.; Fan, H.; Lan-Ying, H.; et al. Enhanced antioxidant activity is modulated by hydrogen sulfide antagonizing ethylene in tomato fruit ripening. J. Agric. Food Chem. 2018, 66, 10380–10387. [Google Scholar]
- Ziogas, V.; Molassiotis, A.; Fotopoulos, V.; Tanou, G. Hydrogen sulfide: A potent tool in postharvest fruit biology and possible mechanism of action. Front. Plant Sci. 2018, 9, 1375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gulfishan, M.; Jahan, A.; Bhat, T.A.; Sahab, D. Plant senescence and organ abscission. In Senescence Signalling and Control in Plants; Bhat, T.A., Ed.; Academic Press: Aligarh, India, 2019; pp. 255–272. [Google Scholar]
- Yasong, C.; Qingfu, C.; Dongao, H.; Guihaia, L.H.J. Research progress on molecular mechanism of plant falling flowers and fruits. Guihaia 2018, 38, 1234–1247. [Google Scholar]
- Olsson, V.; Butenko, M.A. Abscission in plants. Curr. Biol. 2018, 28, 329–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patharkar, O.R.; Walker, J.C. Advances in abscission signaling. J. Exp. Bot 2018, 69, 733–740. [Google Scholar] [CrossRef]
- Taylor, J.E.; Whitelaw, C.A. Signals in abscission. New Phytol. 2001, 151, 323–339. [Google Scholar] [CrossRef]
- Botton, A.; Ruperti, B. The yes and no of the ethylene involvement in abscission. Plants 2019, 8, 187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meir, S.; Philosoph-Hadas, S.; Riov, J.; Tucker, M.L.; Patterson, S.E.; Roberts, J.A. Re-evaluation of the ethylene-dependent and -independent pathways in the regulation of floral and organ abscission. J. Exp. Bot. 2019, 70, 1461–1467. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.Q.; Wei, P.C.; Tan, F.; Yu, M.; Zhang, X.Y.; Chen, Q.J.; Wang, X.C. The transcription factor AtDOF4.7 is involved in ethylene- and IDA-mediated organ abscission in Arabidopsis. Front. Plant Sci. 2016, 7, 863. [Google Scholar] [CrossRef] [Green Version]
- Yamasaki, H.; Ogura, M.P.; Kingjoe, K.A.; Cohen, M.F. d-cysteine-induced rapid root abscission in the water fern Azolla Pinnata: Implications for the linkage between d-amino acid and reactive sulfur species (RSS) in plant environmental responses. Antioxidants 2019, 8, 411. [Google Scholar] [CrossRef] [Green Version]
- Cutler, S.R.; Rodriguez, P.L.; Finkelstein, R.R.; Abrams, S.R. Abscisic acid: Emergence of a core signaling network. Annu. Rev. Plant Biol. 2010, 61, 651–679. [Google Scholar] [CrossRef] [Green Version]
- Jin, Z.P.; Xue, S.W.; Luo, Y.A.; Tian, B.H.; Fang, H.H.; Li, H.; Pei, Y.X. Hydrogen sulfide interacting with abscisic acid in stomatal regulation responses to drought stress in Arabidopsis. Plant Physiol. Biochem. 2013, 62, 41–46. [Google Scholar] [CrossRef]
- Li, Z.G.; Jin, J.Z. Hydrogen sulfide partly mediates abscisic acid-induced heat tolerance in tobacco (Nicotiana tabacum L.) suspension cultured cells. Plant Cell Tiss. Org. 2016, 125, 207–214. [Google Scholar] [CrossRef]
- Li, T.T.; Li, Z.R.; Hu, K.D.; Hu, L.Y.; Chen, X.Y.; Li, Y.H.; Yang, Y.; Yang, F.; Zhang, H. Hydrogen sulfide alleviates kiwifruit ripening and senescence by antagonizing effect of ethylene. Hortscience 2017, 52, 1556–1562. [Google Scholar] [CrossRef] [Green Version]
- Cheng, W.; Zhang, L.; Jiao, C.J.; Su, M.; Yang, T.; Zhou, L.; Peng, R.Y.; Wang, R.; Wang, C.Y. Hydrogen sulfide alleviates hypoxia-induced root tip death in Pisum sativum. Plant Physiol. Biochem. 2013, 70, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.D. Auxin biosynthesis and its role in plant development. Annu. Rev. Plant Biol. 2010, 61, 49–64. [Google Scholar] [CrossRef] [Green Version]
- Shi, H.T.; Ye, T.T.; Han, N.; Bian, H.W.; Liu, X.D.; Chan, Z.L. Hydrogen sulfide regulates abiotic stress tolerance and biotic stress resistance in Arabidopsis. J. Integr. Plant Biol. 2015, 57, 628–640. [Google Scholar] [CrossRef]
- Zhang, X.W.; Liu, F.J.; Zhai, J.; Li, F.D.; Bi, H.G.; Ai, X.Z. Auxin acts as a downstream signaling molecule involved in hydrogen sulfide-induced chilling tolerance in cucumber. Planta 2020, 251, 1–19. [Google Scholar] [CrossRef]
- Achard, P.; Genschik, P. Releasing the brakes of plant growth: How GAs shutdown DELLA proteins. J. Exp. Bot. 2009, 60, 1085–1092. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Dou, W.; Jiang, C.X.; Wei, Z.J.; Liu, J.; Jones, R.L. Hydrogen sulfide stimulates β-amylase activity during early stages of wheat grain germination. Plant Signal. Behav. 2010, 5, 1559–2324. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.J.; Zhang, C.; Lai, D.W.; Sun, Y.; Samma, M.K.; Zhang, J.; Shen, W.B. Hydrogen sulfide delays GA-triggered programmed cell death in wheat aleurone layers by the modulation of glutathione homeostasis and heme oxygenase-1 expression. J. Plant Physiol. 2014, 171, 53–62. [Google Scholar] [CrossRef]
- Raskin, I. Role of salicylic-acid in plants. Annu. Rev. Plant Phys. 1992, 43, 439–463. [Google Scholar] [CrossRef]
- Li, Z.G.; Xie, L.R.; Li, X.J. Hydrogen sulfide acts as a downstream signal molecule in salicylic acid-induced heat tolerance in maize (Zea mays L.) seedlings. J. Plant Physiol. 2015, 177, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Devoto, A.; Turner, J.G. Regulation of jasmonate-mediated plant responses in Arabidopsis. Ann. Bot. London 2003, 92, 329–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balbi, V.; Devoto, A. Jasmonate signalling network in Arabidopsis thaliana: Crucial regulatory nodes and new physiological scenarios. New Phytol. 2008, 177, 301–318. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Hu, Y.R.; Zhang, G.S.; Jiang, Y.J.; Chen, X.L.; Yu, D.Q. Jasmonate negatively regulates stomatal development in Arabidopsis cotyledons. Plant Physiol. 2018, 176, 2871–2885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, G.B.; Zhou, L.J.; Wang, Y.Y.; Zhang, G.S.; Chen, X.L. Hydrogen sulfide acts downstream of jasmonic acid to inhibit stomatal development in Arabidopsis. Planta 2020, 251, 42. [Google Scholar] [CrossRef]
- Zhang, P.; Luo, Q.; Wang, R.L.; Xu, J. Hydrogen sulfide toxicity inhibits primary root growth through the ROS-NO pathway. Sci. Rep. UK 2017, 7, 868. [Google Scholar] [CrossRef]
- Jaffrey, S.R.; Erdjument-Bromage, H.; Ferris, C.D.; Tempst, P.; Snyder, S.H. Protein S-nitrosylation: A physiological signal for neuronal nitric oxide. Nat. Cell Biol. 2001, 3, 193–197. [Google Scholar] [CrossRef]
- Aroca, A.; Serna, A.; Gotor, C.; Romero, L.C. S-Sulfhydration: A cysteine posttranslational modification in plant systems. Plant Physiol. 2015, 168, 334–342. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.G.; Yi, X.Y.; Li, Y.T. Effect of pretreatment with hydrogen sulfide donor sodium hydrosulfide on heat tolerance in relation to antioxidant system in maize (Zea mays) seedlings. Biologia 2014, 69, 1001–1009. [Google Scholar] [CrossRef]
- Uchida, A.; Jagendorf, A.T.; Hibino, T.; Takabe, T.; Takabe, T. Effects of hydrogen peroxide and nitric oxide on both salt and heat stress tolerance in rice. Plant Sci. 2002, 163, 515–523. [Google Scholar] [CrossRef]
- Christou, A.; Filippou, P.; Manganaris, G.A.; Fotopoulos, V. Sodium hydrosulfide induces systemic thermotolerance to strawberry plants through transcriptional regulation of heat shock proteins and aquaporin. BMC Plant Biol. 2014, 14, 42. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Xu, X.Y.; Chen, L.Q.; Yang, J.L.; Zheng, S.J. Nitric oxide exacerbates Al-induced inhibition of root elongation in rice bean by affecting cell wall and plasma membrane properties. Phytochemistry 2012, 76, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.Q.; Zhang, J.H.; Sun, L.M.; Zhu, L.F.; Abliz, B.; Hu, W.J.; Zhong, C.; Bai, Z.G.; Sajid, H.; Cao, X.C.; et al. Hydrogen sulfide alleviates aluminum toxicity via decreasing apoplast and symplast al contents in rice. Front. Plant Sci. 2018, 9, 294. [Google Scholar] [CrossRef] [Green Version]
- Sun, C.L.; Lu, L.L.; Yu, Y.; Liu, L.J.; Hu, Y.; Ye, Y.Q.; Jin, C.W.; Lin, X.Y. Decreasing methylation of pectin caused by nitric oxide leads to higher aluminium binding in cell walls and greater aluminium sensitivity of wheat roots. J. Exp. Bot. 2016, 67, 979–989. [Google Scholar] [CrossRef] [Green Version]
- Pucciariello, C.; Perata, P. New insights into reactive oxygen species and nitric oxide signalling under low oxygen in plants. Plant Cell Environ. 2017, 40, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Peng, R.Y.; Bian, Z.Y.; Zhou, L.N.; Cheng, W.; Hai, N.; Yang, C.Q.; Yang, T.; Wang, X.Y.; Wang, C.Y. Hydrogen sulfide enhances nitric oxide-induced tolerance of hypoxia in maize (Zea mays L.). Plant Cell Rep. 2016, 35, 2325–2340. [Google Scholar] [CrossRef] [PubMed]
- Xuan, W.; Zhu, F.Y.; Xu, S.; Huang, B.K.; Ling, T.F.; Qi, J.Y.; Ye, M.B.; Shen, W.B. The heme oxygenase/carbon monoxide system is involved in the auxin-induced cucumber adventitious rooting process. Plant Physiol. 2008, 148, 881–893. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.T.; Li, M.Y.; Cui, W.T.; Lu, W.; Shen, W.B. Haem oxygenase-1 is involved in hydrogen sulfide-induced cucumber adventitious root formation. J. Plant Growth Regul. 2012, 31, 519–528. [Google Scholar] [CrossRef]
- Li, Z.G.; Gong, M.; Xie, H.; Yang, L.; Li, J. Hydrogen sulfide donor sodium hydrosulfide-induced heat tolerance in tobacco (Nicotiana tabacum L.) suspension cultured cells and involvement of Ca2+ and calmodulin. Plant Sci. 2012, 185, 185–189. [Google Scholar] [CrossRef]
- Li, Z.G.; Long, W.B.; Yang, S.Z.; Wang, Y.C.; Tang, J.H.; Wen, L.; Zhu, B.Y.; Min, X. Endogenous hydrogen sulfide regulated by calcium is involved in thermotolerance in tobacco Nicotiana tabacum L. suspension cell cultures. Acta Physiol. Plant 2015, 37, 1–11. [Google Scholar] [CrossRef]
- Fang, H.H.; Jing, T.; Liu, Z.Q.; Zhang, L.P.; Jin, Z.P.; Pei, Y.X. Hydrogen sulfide interacts with calcium signaling to enhance the chromium tolerance in Setaria italica. Cell Calcium 2014, 56, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Z.J.; Jing, T.; Jin, Z.P.; Liang, Y.L.; Zhang, L.P.; Liu, Z.Q.; Liu, D.M.; Pei, Y.X. CDPKs enhance Cd tolerance through intensifying H2S signal in Arabidopsis thaliana. Plant Soil 2016, 398, 99–110. [Google Scholar] [CrossRef]
- Zhu, J.K. Regulation of ion homeostasis under salt stress. Curr. Opin. Plant Biol. 2003, 6, 441–445. [Google Scholar] [CrossRef]
- Deng, Y.Q.; Bao, J.; Yuan, F.; Liang, X.; Feng, Z.T.; Wang, B.S. Exogenous hydrogen sulfide alleviates salt stress in wheat seedlings by decreasing Na+ content. Plant Growth Regul. 2016, 79, 391–399. [Google Scholar] [CrossRef]
- Zhao, N.; Zhu, H.P.; Zhang, H.P.; Sun, J.; Zhou, J.C.; Deng, C.; Zhang, Y.H.; Zhao, F.; Zhou, X.Y.; Lu, C.F.; et al. Hydrogen sulfide mediates K+ and Na+ homeostasis in the roots of salt-resistant and salt-sensitive poplar species subjected to NaCl stress. Front. Plant Sci. 2018, 9, 1366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mostofa, M.G.; Saegusa, D.; Fujita, M.; Tran, L.S.P. Hydrogen sulfide regulates salt tolerance in rice by maintaining Na+/K+ balance, mineral homeostasis and oxidative metabolism under excessive salt stress. Front. Plant Sci. 2015, 6, 1055. [Google Scholar] [CrossRef] [Green Version]
- Li, J.S.; Jia, H.L.; Wang, J.; Cao, Q.H.; Wen, Z.C. Hydrogen sulfide is involved in maintaining ion homeostasis via regulating plasma membrane Na+/H+ antiporter system in the hydrogen peroxide-dependent manner in salt-stress Arabidopsis thaliana root. Protoplasma 2014, 251, 899–912. [Google Scholar] [CrossRef]
- Paul, B.D.; Snyder, S.H. H2S signalling through protein sulfhydration and beyond. Nat. Rev. Mol. Cell Biol. 2012, 13, 499–507. [Google Scholar] [CrossRef]
- Aroca, A.; Gotor, C.; Romero, L.C. Hydrogen sulfide signaling in plants: Emerging roles of protein persulfidation. Front. Plant Sci. 2018, 9, 1369. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.Q.; Li, Y.W.; Cao, C.Y.; Liang, S.; Ma, Y.S.; Liu, X.; Pei, Y.X. The role of H2S in low temperature-induced cucurbitacin C increases in cucumber. Plant Mol. Biol. 2019, 99, 535–544. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xuan, L.; Li, J.; Wang, X.; Wang, C. Crosstalk between Hydrogen Sulfide and Other Signal Molecules Regulates Plant Growth and Development. Int. J. Mol. Sci. 2020, 21, 4593. https://doi.org/10.3390/ijms21134593
Xuan L, Li J, Wang X, Wang C. Crosstalk between Hydrogen Sulfide and Other Signal Molecules Regulates Plant Growth and Development. International Journal of Molecular Sciences. 2020; 21(13):4593. https://doi.org/10.3390/ijms21134593
Chicago/Turabian StyleXuan, Lijuan, Jian Li, Xinyu Wang, and Chongying Wang. 2020. "Crosstalk between Hydrogen Sulfide and Other Signal Molecules Regulates Plant Growth and Development" International Journal of Molecular Sciences 21, no. 13: 4593. https://doi.org/10.3390/ijms21134593
APA StyleXuan, L., Li, J., Wang, X., & Wang, C. (2020). Crosstalk between Hydrogen Sulfide and Other Signal Molecules Regulates Plant Growth and Development. International Journal of Molecular Sciences, 21(13), 4593. https://doi.org/10.3390/ijms21134593