MnO2 Heterostructure on Carbon Nanotubes as Cathode Material for Aqueous Zinc-Ion Batteries
Abstract
:1. Introduction
2. Results and Discussion
2.1. Material Characterization
2.1.1. X-ray Diffraction (XRD)
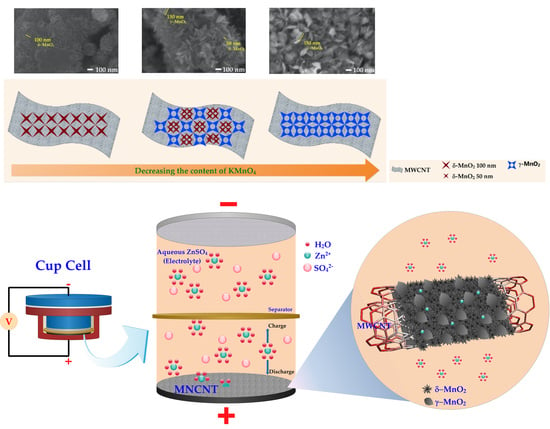
2.1.2. Field Emission Scanning Electron Microscope (FESEM)
2.2. Electrochemical Performances
2.2.1. Battery System
2.2.2. Electrochemical Performances
2.2.3. Pseudocapacitive Behavior
3. Materials and Methods
3.1. Materials
3.2. Preparation of δ-MnO2 and MnO2 Heterostructure/MWCNTs (MNH-CNT)
3.3. Electrodes and Cup Cell Preparation
3.4. Materials Characterization
3.5. Electrochemical Performances
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ZIB | Zinc-ion battery |
| MNH-CNT | MnO2 heterostructure on multi-walled carbon nanotubes |
| MN-CNT | MnO2 on multi-walled carbon nanotubes |
| MN-CNT6040 | MN-CNT having MnO2 and MWCNT ratio of 60:40 |
| MN-CNT7525 | MN-CNT having MnO2 and MWCNT ratio of 75:25 |
| MN-CNT9010 | MN-CNT having MnO2 and MWCNT ratio of 90:10 |
| MIB | Metal-ion battery |
| LIB | Lithium-ion battery |
| SHE | Standard hydrogen potential |
| CNT | Carbon nanotube |
| SWCNT | Single-walled carbon nanotube |
| MWCNT | Multi-walled carbon nanotube |
| XRD | X-ray diffraction |
| FESEM | Field emission scanning electron microscope |
| TEM-EDS | Transmission electron microscope with energy dispersive spectroscopy |
| CV | Cyclic voltammetry |
| CE | Coulombic efficiency |
| OLC | Onion-like carbon |
| DI water | Deionized water |
References
- Corpuz, R.D.; De Juan, L.M.Z.; Praserthdam, S.; Pornprasertsuk, R.; Yonezawa, T.; Nguyen, M.T.; Kheawhom, S. Annealing induced a well-ordered single crystal δ-MnO2 and its electrochemical performance in zinc-ion battery. Sci. Rep. 2019, 9, 15107. [Google Scholar] [CrossRef] [Green Version]
- De Juan-Corpuz, L.M.; Corpuz, R.D.; Somwangthanaroj, A.; Nguyen, M.T.; Yonezawa, T.; Ma, J.; Kheawhom, S. Binder-free centimeter-long V2O5 nanofibers on carbon cloth as cathode material for zinc-ion batteries. Energies 2019, 13, 31. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Mou, J.; Liu, W.; Wang, X.; Dong, L.; Kang, F.; Xu, C. Novel insights into energy storage mechanism of aqueous rechargeable Zn/MnO2 batteries with participation of Mn2+. Nano-Micro Lett. 2019, 11, 49. [Google Scholar] [CrossRef] [Green Version]
- Leisegang, T.; Meutzner, F.; Zschornak, M.; Münchgesang, W.; Schmid, R.; Nestler, T.; Eremin, R.A.; Kabanov, A.A.; Blatov, V.A.; Meyer, D.C. The aluminum-ion battery: A sustainable and seminal concept? Front. Chem. 2019, 7, 268. [Google Scholar] [CrossRef]
- Su, B.; Zhang, J.; Fujita, M.; Zhou, W.; Sit, P.H.-L.; Yu, D.Y.W. Na2SeO3: A Na-ion battery positive electrode material with high capacity. J. Electrochem. Soc. 2019, 166, A5075–A5080. [Google Scholar] [CrossRef]
- Saw, L.H.; Ye, Y.; Tay, A.A.O. Integration issues of lithium-ion battery into electric vehicles battery pack. J. Clean. Prod. 2016, 113, 1032–1045. [Google Scholar] [CrossRef]
- Rao, Z.; Wang, S.; Zhang, G. Simulation and experiment of thermal energy management with phase change material for ageing LiFePO4 power battery. Energy Convers. Manag. 2011, 52, 3408–3414. [Google Scholar] [CrossRef]
- Palaniyandy, N.; Kebede, M.A.; Raju, K.; Ozoemena, K.I.; le Roux, L.; Mathe, M.K.; Jayaprakasam, R. α-MnO2 nanorod/onion-like carbon composite cathode material for aqueous zinc-ion battery. Mater. Chem. Phys. 2019, 230, 258–266. [Google Scholar] [CrossRef]
- Qin, M.; Liu, W.; Shan, L.; Fang, G.; Cao, X.; Liang, S.; Zhou, J. Construction of V2O5/NaV6O15 biphase composites as aqueous zinc-ion battery cathode. J. Electroanal. Chem. 2019, 847, 113246. [Google Scholar] [CrossRef]
- Ould Ely, T.; Kamzabek, D.; Chakraborty, D. Batteries safety: Recent progress and current challenges. Front. Energy Res. 2019, 7. [Google Scholar] [CrossRef]
- Wu, X.; Song, K.; Zhang, X.; Hu, N.; Li, L.; Li, W.; Zhang, L.; Zhang, H. Safety issues in lithium ion batteries: Materials and cell design. Front. Energy Res. 2019, 7. [Google Scholar] [CrossRef] [Green Version]
- Kao-ian, W.; Pornprasertsuk, R.; Thamyongkit, P.; Maiyalagan, T.; Kheawhom, S. Rechargeable zinc-ion battery based on choline chloride-urea deep eutectic solvent. J. Electrochem. Soc. 2019, 166, A1063–A1069. [Google Scholar] [CrossRef]
- Corpuz, R.D.; De Juan-Corpuz, L.M.; Nguyen, M.T.; Yonezawa, T.; Wu, H.-L.; Somwangthanaroj, A.; Kheawhom, S. Binder-free α-MnO2 nanowires on carbon cloth as cathode material for zinc-ion batteries. Int. J. Mol. Sci. 2020, 21, 3113. [Google Scholar] [CrossRef]
- Kundu, D.; Hosseini Vajargah, S.; Wan, L.; Adams, B.; Prendergast, D.; Nazar, L.F. Aqueous vs. nonaqueous Zn-ion batteries: Consequences of the desolvation penalty at the interface. Energy Environ. Sci. 2018, 11, 881–892. [Google Scholar] [CrossRef]
- Alfaruqi, M.H.; Islam, S.; Putro, D.Y.; Mathew, V.; Kim, S.; Jo, J.; Kim, S.; Sun, Y.-K.; Kim, K.; Kim, J. Structural transformation and electrochemical study of layered MnO2 in rechargeable aqueous zinc-ion battery. Electrochim. Acta 2018, 276, 1–11. [Google Scholar] [CrossRef]
- Li, H.; Ma, L.; Han, C.; Wang, Z.; Liu, Z.; Tang, Z.; Zhi, C. Advanced rechargeable zinc-based batteries: Recent progress and future perspectives. Nano Energy 2019, 62, 550–587. [Google Scholar] [CrossRef]
- Lao-atiman, W.; Julaphatachote, T.; Boonmongkolras, P.; Kheawhom, S. Printed Transparent Thin Film Zn-MnO2 Battery. J. Electrochem. Soc. 2017, 164, A859–A863. [Google Scholar] [CrossRef]
- Hosseini, S.; Lao-atiman, W.; Han, S.J.; Arpornwichanop, A.; Yonezawa, T.; Kheawhom, S. Discharge performance of zinc-air flow batteries under the effects of sodium dodecyl sulfate and pluronic F-127. Sci. Rep. 2018, 8, 14909. [Google Scholar] [CrossRef] [Green Version]
- Hosseini, S.; Han, S.J.; Arponwichanop, A.; Yonezawa, T.; Kheawhom, S. Ethanol as an electrolyte additive for alkaline zinc-air flow batteries. Sci. Rep. 2018, 8, 11273. [Google Scholar] [CrossRef] [Green Version]
- Ma, N.; Wu, P.; Wu, Y.; Jiang, D.; Lei, G. Progress and perspective of aqueous zinc-ion battery. Funct. Mater. Lett. 2019, 12, 1930003. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Wang, Y. Recent Progress on Zinc-Ion Rechargeable Batteries. Nano-Micro Lett. 2019, 11, 90. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Li, J.; Jin, X.; Han, Y.; Lin, Y.; Lei, Z.; Wang, S.; Qin, L.; Jiao, S.; Cao, R. A Hollow-structured manganese oxide cathode for stable Zn-MnO2 batteries. Nanomaterials 2018, 8, 301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, B.; Xu, C.; Wu, C.; Dong, L.; Li, J.; Kang, F. Manganese sesquioxide as cathode material for multivalent zinc ion battery with high capacity and long cycle life. Electrochim. Acta 2017, 229, 422–428. [Google Scholar] [CrossRef]
- Lan, B.; Peng, Z.; Chen, L.; Tang, C.; Dong, S.; Chen, C.; Zhou, M.; Chen, C.; An, Q.; Luo, P. Metallic silver doped vanadium pentoxide cathode for aqueous rechargeable zinc ion batteries. J. Alloys Compd. 2019, 787, 9–16. [Google Scholar] [CrossRef]
- Ming, J.; Guo, J.; Xia, C.; Wang, W.; Alshareef, H.N. Zinc-ion batteries: Materials, mechanisms, and applications. Mater. Sci. Eng. R Rep. 2019, 135, 58–84. [Google Scholar] [CrossRef]
- Yang, D.; Tan, H.; Rui, X.; Yu, Y. Electrode materials for rechargeable zinc-ion and zinc-air batteries: Current status and future perspectives. Electrochemical Energy Reviews 2019, 2, 395–427. [Google Scholar] [CrossRef]
- Tang, B.; Shan, L.; Liang, S.; Zhou, J. Issues and opportunities facing aqueous zinc-ion batteries. Energy Environ. Sci. 2019, 12, 3288–3304. [Google Scholar] [CrossRef]
- Guo, C.; Liu, H.; Li, J.; Hou, Z.; Liang, J.; Zhou, J.; Zhu, Y.; Qian, Y. Ultrathin δ-MnO2 nanosheets as cathode for aqueous rechargeable zinc ion battery. Electrochim. Acta 2019, 304, 370–377. [Google Scholar] [CrossRef]
- Wang, C.; Zeng, Y.; Xiao, X.; Wu, S.; Zhong, G.; Xu, K.; Wei, Z.; Su, W.; Lu, X. γ-MnO2 nanorods/graphene composite as efficient cathode for advanced rechargeable aqueous zinc-ion battery. J. Energy Chem. 2020, 43, 182–187. [Google Scholar] [CrossRef] [Green Version]
- Thapa, A.K.; Pandit, B.; Thapa, R.; Luitel, T.; Paudel, H.S.; Sumanasekera, G.; Sunkara, M.K.; Gunawardhana, N.; Ishihara, T.; Yoshio, M. Synthesis of mesoporous birnessite-MnO2 composite as a cathode electrode for lithium battery. Electrochim. Acta 2014, 116, 188–193. [Google Scholar] [CrossRef]
- Wang, J.-W.; Chen, Y.; Chen, B.-Z. Synthesis and control of high-performance MnO2/carbon nanotubes nanocomposites for supercapacitors. J. Alloys Compd. 2016, 688, 184–197. [Google Scholar] [CrossRef]
- Khamsanga, S.; Pornprasertsuk, R.; Yonezawa, T.; Mohamad, A.A.; Kheawhom, S. δ-MnO2 nanoflower/graphite cathode for rechargeable aqueous zinc ion batteries. Sci. Rep. 2019, 9, 8441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Hu, Z.A.; An, N.; Yang, Y.Y.; Li, Z.M.; Wu, H.Y. Facile Synthesis of MnO2/CNTs Composite for supercapacitor electrodes with long cycle stability. J. Phys. Chem. 2014, 118, 22865–22872. [Google Scholar] [CrossRef]
- Wang, H.; Peng, C.; Peng, F.; Yu, H.; Yang, J. Facile synthesis of MnO2/CNT nanocomposite and its electrochemical performance for supercapacitors. Mater. Sci. Eng. 2011, 176, 1073–1078. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, Y.; Li, S.; Sun, J.; Hou, B. Manganese dioxide-coated carbon nanotubes as an improved cathodic catalyst for oxygen reduction in a microbial fuel cell. J. Power Sources 2011, 196, 9284–9289. [Google Scholar] [CrossRef]
- Chou, S.-L.; Wang, J.-Z.; Chew, S.-Y.; Liu, H.-K.; Dou, S.-X. Electrodeposition of MnO2 nanowires on carbon nanotube paper as free-standing, flexible electrode for supercapacitors. Electrochem. Commun. 2008, 10, 1724–1727. [Google Scholar] [CrossRef]
- Yang, J.; Ma, M.; Sun, C.; Zhang, Y.; Huang, W.; Dong, X. Hybrid NiCo2S4@MnO2 heterostructures for high-performance supercapacitor electrodes. J. Mater. Chem. 2015, 3, 1258–1264. [Google Scholar] [CrossRef]
- Liu, L.; Fang, L.; Wu, F.; Hu, J.; Zhang, S.; Luo, H.; Hu, B.; Zhou, M. Self-supported core-shell heterostructure MnO2/NiCo-LDH composite for flexible high-performance supercapacitor. J. Alloys Compd. 2020, 824, 153929. [Google Scholar] [CrossRef]
- Peng, R.; Zhang, H.; Gui, L.; Zheng, Y.; Wu, Z.; Luo, Y.; Yu, P. Construction of 0D CeO2/2D MnO2 heterostructure with high electrochemical performance. Electrochim. Acta 2019, 319, 95–100. [Google Scholar] [CrossRef]
- Alfaruqi, M.H.; Gim, J.; Kim, S.; Song, J.; Pham, D.T.; Jo, J.; Xiu, Z.; Mathew, V.; Kim, J. A layered δ-MnO2 nanoflake cathode with high zinc-storage capacities for eco-friendly battery applications. Electrochem. Commun. 2015, 60, 121–125. [Google Scholar] [CrossRef]
- Zhao, J.; Tao, Z.; Liang, J.; Chen, J. Facile synthesis of nanoporous γ-MnO2 Structures and their application in rechargeable Li-ion batteries. Cryst. Growth Des. 2008, 8, 2799–2805. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Wang, L.; Zhang, C.; He, H. Catalytic oxidation of formaldehyde over manganese oxides with different crystal structures. Catal. Sci. Technol. 2015, 5, 2305–2313. [Google Scholar] [CrossRef]
- Misnon, I.I.; Aziz, R.A.; Zain, N.K.M.; Vidhyadharan, B.; Krishnan, S.G.; Jose, R. High performance MnO2 nanoflower electrode and the relationship between solvated ion size and specific capacitance in highly conductive electrolytes. Mater. Res. Bull. 2014, 57, 221–230. [Google Scholar] [CrossRef] [Green Version]
- Hayes, A.C.; Kruus, P.; Adams, W.A. Raman spectroscopic study of aqueous (NH4)2SO4 and ZnSO4 solutions. J. Solution Chem. 1984, 13, 61–75. [Google Scholar] [CrossRef]
- Zhao, J.; Ren, H.; Liang, Q.; Yuan, D.; Xi, S.; Wu, C.; Manalastas, W.; Ma, J.; Fang, W.; Zheng, Y.; et al. High-performance flexible quasi-solid-state zinc-ion batteries with layer-expanded vanadium oxide cathode and zinc/stainless steel mesh composite anode. Nano Energy 2019, 62, 94–102. [Google Scholar] [CrossRef]
- Kitchaev, D.A.; Peng, H.; Liu, Y.; Sun, J.; Perdew, J.P.; Ceder, G. Energetics of MnO2 polymorphs in density functional theory. Phys. Rev. B 2016, 93, 045132. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.Y.; Wessells, C.D.; Huggins, R.A.; Cui, Y. Highly reversible open framework nanoscale electrodes for divalent ion batteries. Nano Lett. 2013, 13, 5748–5752. [Google Scholar] [CrossRef]
- Lee, J.; Ju, J.B.; Cho, W.I.; Cho, B.W.; Oh, S.H. Todorokite-type MnO2 as a zinc-ion intercalating material. Electrochim. Acta 2013, 112, 138–143. [Google Scholar] [CrossRef]
- Wei, C.; Xu, C.; Li, B.; Du, H.; Kang, F. Preparation and characterization of manganese dioxides with nano-sized tunnel structures for zinc ion storage. J. Phys. Chem. Solids 2012, 73, 1487–1491. [Google Scholar] [CrossRef]
- Islam, S.; Alfaruqi, M.H.; Song, J.; Kim, S.; Pham, D.T.; Jo, J.; Kim, S.; Mathew, V.; Baboo, J.P.; Xiu, Z.; et al. Carbon-coated manganese dioxide nanoparticles and their enhanced electrochemical properties for zinc-ion battery applications. J. Energy Chem. 2017, 26, 815–819. [Google Scholar] [CrossRef] [Green Version]
- Sun, W.; Wang, F.; Hou, S.; Yang, C.; Fan, X.; Ma, Z.; Gao, T.; Han, F.; Hu, R.; Zhu, M.; et al. Zn/MnO2 Battery Chemistry With H+ and Zn2+ Coinsertion. J. Am. Chem. Soc. 2017, 139, 9775–9778. [Google Scholar] [CrossRef] [PubMed]
- Alfaruqi, M.H.; Islam, S.; Gim, J.; Song, J.; Kim, S.; Pham, D.T.; Jo, J.; Xiu, Z.; Mathew, V.; Kim, J. A high surface area tunnel-type α-MnO2 nanorod cathode by a simple solvent-free synthesis for rechargeable aqueous zinc-ion batteries. Chem. Phys. Lett. 2016, 650, 64–68. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, M.; Wu, X.; Wu, X.; Zeng, F.; Li, Y.; Duan, S.; Fan, D.; Yang, Y.; Wu, X. The excellent electrochemical performances of ZnMn2O4/Mn2O3: The composite cathode material for potential aqueous zinc ion batteries. J. Electroanal. Chem. 2019, 832, 69–74. [Google Scholar] [CrossRef]
- Pan, H.; Shao, Y.; Yan, P.; Cheng, Y.; Han, K.S.; Nie, Z.; Wang, C.; Yang, J.; Li, X.; Bhattacharya, P.; et al. Reversible aqueous zinc/manganese oxide energy storage from conversion reactions. Nat. Energy 2016, 1, 16039. [Google Scholar] [CrossRef]
- Lindström, H.; Södergren, S.; Solbrand, A.; Rensmo, H.; Hjelm, J.; Hagfeldt, A.; Lindquist, S.-E. Li+ ion insertion in TiO2 (anatase). 2. Voltammetry on nanoporous films. J. Phys. Chem. B 1997, 101, 7717–7722. [Google Scholar] [CrossRef]
- Wang, J.; Polleux, J.; Lim, J.; Dunn, B. Pseudocapacitive contributions to electrochemical energy storage in TiO2 (anatase) nanoparticles. J. Phys. Chem. C 2007, 111, 14925–14931. [Google Scholar] [CrossRef]
- Kim, H.-S.; Cook, J.B.; Lin, H.; Ko, J.S.; Tolbert, S.H.; Ozolins, V.; Dunn, B. Oxygen vacancies enhance pseudocapacitive charge storage properties of MoO3−x. Nat. Mater. 2017, 16, 454–460. [Google Scholar] [CrossRef]
- Wan, F.; Zhang, L.; Dai, X.; Wang, X.; Niu, Z.; Chen, J. Aqueous rechargeable zinc/sodium vanadate batteries with enhanced performance from simultaneous insertion of dual carriers. Nat. Commun. 2018, 9, 1656. [Google Scholar] [CrossRef] [Green Version]
- Sun, G.; Jin, X.; Yang, H.; Gao, J.; Qu, L. An aqueous Zn–MnO2 rechargeable microbattery. J. Mater. Chem. A 2018, 6, 10926–10931. [Google Scholar] [CrossRef]
- Xia, C.; Guo, J.; Lei, Y.; Liang, H.; Zhao, C.; Alshareef, H.N. Rechargeable aqueous zinc-ion battery based on porous framework zinc pyrovanadate intercalation cathode. Adv. Mater. 2018, 30, 1705580. [Google Scholar] [CrossRef]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khamsanga, S.; Nguyen, M.T.; Yonezawa, T.; Thamyongkit, P.; Pornprasertsuk, R.; Pattananuwat, P.; Tuantranont, A.; Siwamogsatham, S.; Kheawhom, S. MnO2 Heterostructure on Carbon Nanotubes as Cathode Material for Aqueous Zinc-Ion Batteries. Int. J. Mol. Sci. 2020, 21, 4689. https://doi.org/10.3390/ijms21134689
Khamsanga S, Nguyen MT, Yonezawa T, Thamyongkit P, Pornprasertsuk R, Pattananuwat P, Tuantranont A, Siwamogsatham S, Kheawhom S. MnO2 Heterostructure on Carbon Nanotubes as Cathode Material for Aqueous Zinc-Ion Batteries. International Journal of Molecular Sciences. 2020; 21(13):4689. https://doi.org/10.3390/ijms21134689
Chicago/Turabian StyleKhamsanga, Sonti, Mai Thanh Nguyen, Tetsu Yonezawa, Patchanita Thamyongkit, Rojana Pornprasertsuk, Prasit Pattananuwat, Adisorn Tuantranont, Siwaruk Siwamogsatham, and Soorathep Kheawhom. 2020. "MnO2 Heterostructure on Carbon Nanotubes as Cathode Material for Aqueous Zinc-Ion Batteries" International Journal of Molecular Sciences 21, no. 13: 4689. https://doi.org/10.3390/ijms21134689
APA StyleKhamsanga, S., Nguyen, M. T., Yonezawa, T., Thamyongkit, P., Pornprasertsuk, R., Pattananuwat, P., Tuantranont, A., Siwamogsatham, S., & Kheawhom, S. (2020). MnO2 Heterostructure on Carbon Nanotubes as Cathode Material for Aqueous Zinc-Ion Batteries. International Journal of Molecular Sciences, 21(13), 4689. https://doi.org/10.3390/ijms21134689