Lessons from Exploring Chemical Space and Chemical Diversity of Propolis Components
Abstract
:1. Introduction
2. Propolis Components: Chemistry and Geographical Distributions
2.1. Propolis Classification
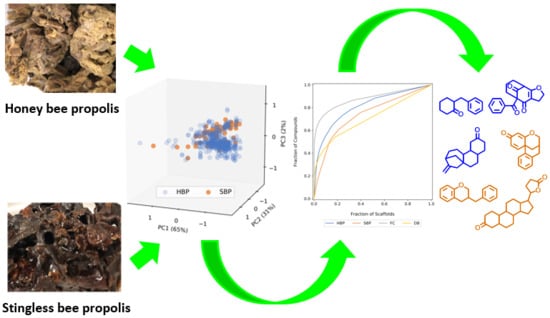
2.1.1. Honey Bee Propolis
2.1.2. Stingless Bee Propolis (Cerumen or Geopropolis)
2.2. Chemical Components of Propolis
2.3. Characteristic Chemical Class of Propolis
3. Physicochemical Property Profiles and Chemical Diversity Analysis of Propolis Components
3.1. Physicochemical Property Profiles
3.2. Structural Diversity
3.2.1. Fingerprint-Based Diversity
3.2.2. Scaffold Diversity
4. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AGE | Advanced glycation endproducts |
| AH1N1 | Influenza A virus subtype H1N1 |
| AP-1 | Activator protein 1 |
| AUC | Area under the cyclic system recovery curve |
| CAPE | Caffeic acid phenyl ester |
| COX-2 | Cyclooxygenase-2 |
| CSR | Cyclic system recovery |
| CXCL2 | Chemokine ligand 2 |
| CYP2E1 | Cytochrome P450 Family 2 Subfamily E Member 1 |
| DB | Drug bank |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| ERK 1/2 | Extracellular signal-regulated protein kinase 1/2 |
| FC | Food chemicals |
| FDA | Food and Drug Administration |
| GC | Gas chromatography |
| GC-MS | Gas chromatography—Mass spectrometry |
| GRP78 | Glucose Regulated Protein 78 |
| HBA | Hydrogen bond acceptor |
| HBD | Hydrogen bond donor |
| HBP | Honey bee propolis |
| HIF-1a | Hypoxia-inducible factor 1-alpha |
| HIV-1 | Human immunodeficiency virus 1 |
| HPLC | High-performance liquid chromatography |
| IL-4 | Interleukin 4 |
| IL-5 | Interleukin 5 |
| IL-13 | Interleukin 13 |
| iNOS | Inducible nitric oxide synthase |
| JAK2 | Janus Kinase 2 |
| JNK | Jun N-terminal kinases |
| LC-MS | Liquid chromatography—Mass spectrometry |
| LogP | Partition coefficient between octanol and water |
| MACCS | Molecular ACCess System |
| MIP-2 | Macrophage inflammatory protein 2 |
| MMP-1 | Matrix metalloproteinase-1 |
| MMP-2 | Matrix metalloproteinase-2 |
| MMP-3 | Matrix metalloproteinase-3 |
| MMP-9 | Matrix metalloproteinase-9 |
| MMP-10 | Matrix metalloproteinase-10 |
| MMP-13 | Matrix metalloproteinase-13 |
| MS | Mass spectrometry |
| NF-κB | Nuclear factor kappa B |
| NMR | Nuclear magnetic resonance |
| NO | Nitric oxide |
| p38 MAPK | p38 mitogen-activated protein kinases |
| PERK | Protein kinase RNA-like endoplasmic reticulum kinase |
| PI3K | Phosphoinositide 3-kinases |
| P-gp | Permeability glycoprotein |
| PPARγ | Peroxisome proliferator-activated receptor gamma |
| RAGE | Receptor for advanced glycation endproducts |
| RB | Rotatable bond |
| ROS | Reactive oxygen species |
| SBP | Stingless bee propolis |
| STAT3 | Signal transducer and activator of transcription 3 |
| TGF-β | Transforming growth factor beta |
| TNF-α | Tumor necrosis factor alpha |
| tPSA | Topological polar surface area |
| TRAIL | Tumor necrosis factor-related apoptosis-inducing ligand |
| VEGF | Vascular endothelial growth factor |
| Wnt | Wingless-related integration site |
References
- Ribeiro da Cunha, B.; Fonseca, L.P.; Calado, C.R.C. Antibiotic discovery: Where have we come from, where do we go? Antibiotics 2019, 8, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cragg, G.M.; Newman, D.J. Natural products: A continuing source of novel drug leads. Biochim. Biophys. Acta 2013, 1830, 3670–3695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogbourne, S.M.; Parsons, P.G. The value of nature’s natural product library for the discovery of new chemical entities: The discovery of ingenol mebutate. Fitoterapia 2014, 98, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Davison, E.K.; Brimble, M.A. Natural product derived privileged scaffolds in drug discovery. Curr. Opin. Chem. Biol. 2019, 52, 1–8. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.L.; Edrada-Ebel, R.; Quinn, R.J. The re-emergence of natural products for drug discovery in the genomics era. Nat. Rev. Drug Discov. 2015, 14, 111–129. [Google Scholar] [CrossRef] [Green Version]
- Lachance, H.; Wetzel, S.; Kumar, K.; Waldmann, H. Charting, navigating, and populating natural product chemical space for drug discovery. J. Med. Chem. 2012, 55, 5989–6001. [Google Scholar] [CrossRef]
- Gerry, C.J.; Schreiber, S.L. Chemical probes and drug leads from advances in synthetic planning and methodology. Nat. Rev. Drug Discov. 2018, 17, 333–352. [Google Scholar] [CrossRef]
- Chávez-Hernández, A.L.; Sánchez-Cruz, N.; Medina-Franco, J.L. A fragment library of natural products and its comparative chemoinformatic characterization. Mol. Inf. 2020, 39, 2000050. [Google Scholar] [CrossRef]
- Lovering, F.; Bikker, J.; Humblet, C. Escape from Flatland: Increasing Saturation as an Approach to Improving Clinical Success. J. Med. Chem. 2009, 52, 6752–6756. [Google Scholar] [CrossRef] [PubMed]
- Ghisalberti, E.L. Propolis: A review. Bee World 1979, 60, 59–84. [Google Scholar] [CrossRef]
- Toreti, V.C.; Sato, H.H.; Pastore, G.M.; Park, Y.K. Recent progress of propolis for its biological and chemical compositions and its botanical origin. Evid. Based Complement. Altern. Med. 2013, 2013, 697390. [Google Scholar] [CrossRef] [PubMed]
- Silva-Carvalho, R.; Baltazar, F.; Almeida-Aguiar, C. Propolis: A complex natural product with a plethora of biological activities that can be explored for drug development. Evid. Based Complement. Altern. Med. 2015, 2015, 206439. [Google Scholar] [CrossRef]
- Castaldo, S.; Capasso, F. Propolis, an old remedy used in modern medicine. Fitoterapia 2002, 73, S1–S6. [Google Scholar] [CrossRef]
- Patel, S. Emerging adjuvant therapy for cancer: Propolis and its constituents. J. Diet. Suppl. 2016, 13, 245–268. [Google Scholar] [CrossRef]
- Aminimoghadamfarouj, N.; Nematollahi, A. Propolis diterpenes as a remarkable bio-source for drug discovery development: A review. Int. J. Mol. Sci. 2017, 18, 1290. [Google Scholar] [CrossRef] [Green Version]
- Pierce, C.H. Composition for Treating Piano Pins and Strings. U.S Patent No. 767,499, 16 August 1904. [Google Scholar]
- Wagh, V.D. Propolis: A wonder bees product and its pharmacological potentials. Adv. Pharmacol. Sci. 2013, 308249. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.; Zhang, C.P.; Wang, K.; Li, G.Q.; Hu, F.L. Recent advances in the chemical composition of propolis. Molecules 2014, 19, 19610–19632. [Google Scholar] [CrossRef] [Green Version]
- Bogdanov, S.; Bankova, V. The Propolis Book, Chapter 1—Propolis: Origin, Production, Composition; Bee Product Science: Muehlethurnen, Switzerland, 2016. [Google Scholar]
- Freires, I.A.; de Alencar, S.M.; Rosalen, P.L. A pharmacological perspective on the use of Brazilian red propolis and its isolated compounds against human diseases. Eur. J. Med. Chem. 2016, 110, 267–279. [Google Scholar] [CrossRef]
- Franchin, M.; Freires, I.A.; Lazarini, J.G.; Nani, B.D.; da Cunha, M.G.; Colón, D.F.; de Alencar, S.M.; Rosalen, P.L. The use of Brazilian propolis for discovery and development of novel anti-inflammatory drugs. Eur. J. Med. Chem. 2018, 153, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Marcucci, M.C. Propolis: Chemical composition, biological properties and therapeutic activity. Apidologie 1995, 26, 83–99. [Google Scholar] [CrossRef]
- Bankova, V.S.; De Castro, S.L.; Marcucci, M.C. Propolis: Recent advances in chemistry and plant origin. Apidologie 2000, 31, 3–15. [Google Scholar] [CrossRef]
- Salatino, A.; Fernandes-Silva, C.C.; Righi, A.A.; Salatino, M.L.F. Propolis research and the chemistry of plant products. Nat. Prod. Rep. 2011, 28, 925–936. [Google Scholar] [CrossRef] [PubMed]
- Miguel, M.G.; Antunes, M.D. Is propolis safe as an alternative medicine? J. Pharm. Bioall. Sci. 2011, 3, 479–495. [Google Scholar] [CrossRef]
- Bankova, V.; Popova, M.; Trusheva, B. The phytochemistry of the honeybee. Phytochemistry 2018, 155, 1–11. [Google Scholar] [CrossRef]
- Lavinas, F.C.; Macedo, E.H.B.C.; Sá, G.B.L.; Amaral, A.C.F.; Silva, J.R.A.; Azevedo, M.; Vieira, B.A.; Domingos, T.F.S.; Vermelho, A.B.; Carneiro, C.S. Brazilian stingless bee propolis and geopropolis: Promising sources of biologically active compounds. Rev. Bras. Farmacogn. 2019, 29, 389–399. [Google Scholar] [CrossRef]
- Popova, M.; Trusheva, B.; Bankova, V. Propolis of stingless bees: A phytochemist’s guide through the jungle of tropical biodiversity. Phytomedicine 2019, in press. [Google Scholar] [CrossRef]
- Martinotti, S.; Ranzato, E. Propolis: A new frontier for wound healing? Burn. Trauma 2015, 3, 9. [Google Scholar] [CrossRef] [Green Version]
- Oryan, A.; Alemzadeh, E.; Moshiri, A. Potential role of propolis in wound healing: Biological properties and therapeutic activities. Biomed. Pharmacother. 2018, 98, 469–483. [Google Scholar] [CrossRef]
- Sanches, M.A.; Pereira, A.M.S.; Serrão, J.E. Pharmacological actions of extracts of propolis of stingless bees (Meliponini). J. Apic. Res. 2017, 56, 50–57. [Google Scholar] [CrossRef]
- Khurshid, Z.; Naseem, M.; Zafar, M.S.; Najeeb, S.; Zohaib, S. Propolis: A natural biomaterial for dental and oral healthcare. J. Dent. Res. Dent. Clin. Dent. Prospect. 2017, 11, 265–274. [Google Scholar]
- Silva, L.M.d.; Souza, P.d.; Jaouni, S.K.A.; Harakeh, S.; Golbabapour, S.; De Andrade, S.F. Propolis and its potential to treat gastrointestinal disorders. Evid. Based Complement. Altern. Med. 2018, 2018, 2035820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradbear, N. Bees and Their Role in Forest Livelihoods: A Guide to the Services Provided by Bees and the Sustainable Harvesting, Processing and Marketing of Their Products; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2009. [Google Scholar]
- Meyer, W.; Ulrich, W. ‘Propolis Bees’ and Their Activities. Bee World 1956, 37, 25–36. [Google Scholar] [CrossRef]
- Sahinler, N.; Kaftanoglu, O. Natural product propolis: Chemical composition. Nat. Prod. Res. 2005, 19, 183–188. [Google Scholar] [CrossRef]
- Shkenderov, S.; Produkti, I.T.P. The bee products, in Bulgarian. Zemizdat 1983, 1–238. [Google Scholar]
- Clarke, M. Australian Propolis Market and Production Potential; AgriFutures Australia: Canberra, Australia, 2019; pp. 1–46. [Google Scholar]
- Hrncir, M.; Jarau, S.; Barth, F.G. Stingless bees (Meliponini): Senses and behavior. J. Comp. Physiol. A 2016, 202, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Miyata, R.; Sahlan, M.; Ishikawa, Y.; Hashimoto, H.; Honda, S.; Kumazawa, S. Propolis components from stingless bees collected on south sulawesi, Indonesia, and their xanthine oxidase inhibitory activity. J. Nat. Prod. 2019, 82, 205–210. [Google Scholar] [CrossRef]
- Velikova, M.; Bankova, V.; Tsvetkova, I.; Kujumgiev, A.; Marcucci, M.C. Antibacterial ent-kaurene from Brazilian propolis of native stingless bees. Fitoterapia 2000, 71, 693–696. [Google Scholar] [CrossRef]
- Ghisalberti, E.L.; Jefferies, P.R.; Lanteri, R.; Matisons, J. Constituents of propolis. Experientia 1978, 34, 157–158. [Google Scholar] [CrossRef]
- Bankova, V.S.; Popov, S.S.; Marekov, N.L. A study on flavonoids of propolis. J. Nat. Prod. 1983, 46, 471–474. [Google Scholar] [CrossRef]
- Yamauchi, R.; Kato, K.; Oida, S.; Kanaeda, J.; Ueno, Y. Benzyl caffeate, an antioxidative compound isolated from propolis. Biosci. Biotechnol. Biochem. 1992, 56, 1321–1322. [Google Scholar] [CrossRef]
- Aga, H.; Shibuya, T.; Sugimoto, T.; Kurimoto, M.; Nakajima, S. Isolation and identification of antimicrobial compounds in Brazilian propolis. Biosci. Biotechnol. Biochem. 1994, 58, 945–946. [Google Scholar] [CrossRef]
- Bankova, V.; Marcucci, M.C.; Simova, S.; Nikolova, N.; Kujumgiev, A.; Popov, S. Antibacterial diterpenic acids from Brazilian propolis. Z. Naturforsch. C 1996, 51, 277–280. [Google Scholar] [CrossRef]
- Bankova, V.; Nikolova, N.; Marcucci, M. A new lignan from Brazilian propolis. Z. Naturforsch. C 1996, 51, 735–737. [Google Scholar] [CrossRef]
- Basnet, P.; Matsushige, K.; Hase, K.; Kadota, S.; Namba, T. Four di-O-caffeoyl quinic acid derivatives from propolis. Potent hepatoprotective activity in experimental liver injury models. Biol. Pharm. Bull. 1996, 19, 1479–1484. [Google Scholar] [CrossRef] [Green Version]
- Tatefuji, T.; Izumi, N.; Ohta, T.; Arai, S.; Ikeda, M.; Kurimoto, M. Isolation and identification of compounds from Brazilian propolis which enhance macrophage spreading and mobility. Biol. Pharm. Bull. 1996, 19, 966–970. [Google Scholar] [CrossRef] [Green Version]
- Matsuno, T.; Matsumoto, Y.; Saito, M.; Morikawa, J. Isolation and characterization of cytotoxic diterpenoid isomers from propolis. Z. Naturforsch. C 1997, 52, 702–704. [Google Scholar] [CrossRef] [Green Version]
- Banskota, A.H.; Tezuka, Y.; Prasain, J.K.; Matsushige, K.; Saiki, I.; Kadota, S. Chemical constituents of Brazilian propolis and their cytotoxic activities. J. Nat. Prod. 1998, 61, 896–900. [Google Scholar] [CrossRef]
- Tazawa, S.; Warashina, T.; Noro, T.; Miyase, T. Studies on the constituents of Brazilian propolis. Chem. Pharm. Bull. 1998, 46, 1477–1479. [Google Scholar] [CrossRef] [Green Version]
- Valcic, S.; Montenegro, G.; Timmermann, B.N. Lignans from Chilean propolis. J. Nat. Prod. 1998, 61, 771–775. [Google Scholar] [CrossRef] [PubMed]
- Christov, R.; Bankova, V.; Tsvetkova, I.; Kujumgiev, A.; Tejera, A.D. Antibacterial furofuran lignans from Canary Islands propolis. Fitoterapia 1999, 70, 89–92. [Google Scholar] [CrossRef]
- Hayashi, K.; Komura, S.; Isaji, N.; Ohishi, N.; Yagi, K. Isolation of antioxidative compounds from Brazilian propolis: 3, 4-dihydroxy-5-prenylcinnamic acid, a novel potent antioxidant. Chem. Pharm. Bull. 1999, 47, 1521–1524. [Google Scholar] [CrossRef] [Green Version]
- Rubio, O.C.; Cuellar, A.C.; Rojas, N.; Castro, H.V.; Rastrelli, L.; Aquino, R. A polyisoprenylated benzophenone from Cuban propolis. J. Nat. Prod. 1999, 62, 1013–1015. [Google Scholar] [CrossRef] [PubMed]
- Tazawa, S.; Warashina, T.; Noro, T. Studies on the constituents of Brazilian propolis. II. Chem. Pharm. Bull. 1999, 47, 1388–1392. [Google Scholar] [CrossRef] [Green Version]
- Banskota, A.H.; Tezuka, Y.; Midorikawa, K.; Matsushige, K.; Kadota, S. Two novel cytotoxic benzofuran derivatives from Brazilian propolis. J. Nat. Prod. 2000, 63, 1277–1279. [Google Scholar] [CrossRef] [PubMed]
- Ito, J.; Chang, F.-R.; Wang, H.-K.; Park, Y.K.; Ikegaki, M.; Kilgore, N.; Lee, K.-H. Anti-AIDS agents. 48. Anti-HIV activity of moronic acid derivatives and the new melliferone-related triterpenoid isolated from Brazilian propolis. J. Nat. Prod. 2001, 64, 1278–1281. [Google Scholar] [CrossRef]
- Kusumoto, T.; Miyamoto, T.; Higuchi, R.; Doi, S.; Sugimoto, H.; Yamada, H. Isolation and structures of two new compounds from the essential oil of Brazilian propolis. Chem. Pharm. Bull. 2001, 49, 1207–1209. [Google Scholar] [CrossRef] [Green Version]
- Popova, M.; Bankova, V.; Spassov, S.; Tsvetkova, I.; Silva, M.V.; Tsartsarova, M.; Naydenski, C. New bioactive chalcones in propolis from El Salvador. Z. Naturfosch. C 2001, 56, 593–596. [Google Scholar] [CrossRef]
- Popova, M.; Bankova, V.; Tsvetkova, I.; Naydenski, C.; Silva, M.V. The first glycosides isolated from propolis: Diterpene rhamnosides. Z. Naturfosch. C 2001, 56, 1108–1111. [Google Scholar] [CrossRef]
- Banskota, A.H.; Nagaoka, T.; Sumioka, L.Y.; Tezuka, Y.; Awale, S.; Midorikawa, K.; Matsushige, K.; Kadota, S. Antiproliferative activity of the Netherlands propolis and its active principles in cancer cell lines. J. Ethnopharmacol. 2002, 80, 67–73. [Google Scholar] [CrossRef]
- Cuesta-Rubio, O.; Frontana-Uribe, B.A.; Ramírez-Apan, T.; Cárdenas, J. Polyisoprenylated benzophenones In Cuban propolis; biological activity of nemorosone. Z. Nat. C 2002, 57, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Takagi, N.; Ikeda, T.; Ono, M.; Nafady, A.M.; Nohara, T.; Sugimoto, H.; Doi, S.; Yamada, H. Two novel long-chain alkanoic acid esters of lupeol from Alecrim-propolis. Chem. Pharm. Bull. 2002, 50, 439–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumazawa, S.; Hayashi, K.; Kajiya, K.; Ishii, T.; Hamasaka, T.; Nakayama, T. Studies of the constituents of Uruguayan propolis. J. Agric. Food Chem. 2002, 50, 4777–4782. [Google Scholar] [CrossRef]
- Usia, T.; Banskota, A.H.; Tezuka, Y.; Midorikawa, K.; Matsushige, K.; Kadota, S. Constituents of Chinese propolis and their antiproliferative activities. J. Nat. Prod. 2002, 65, 673–676. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-N.; Wu, C.-L.; Shy, H.-S.; Lin, J.-K. Cytotoxic prenylflavanones from Taiwanese propolis. J. Nat. Prod. 2003, 66, 503–506. [Google Scholar] [CrossRef]
- Nafady, A.M.; El-Shanawany, M.A.; Mohamed, M.H.; Hassanean, H.A.-H.; Nohara, T.; Yoshimitsu, H.; Ono, M.; Sugimoto, H.; Doi, S.; Sasaki, K. Cyclodextrin-enclosed substances of Brazilian propolis. Chem. Pharm. Bull. 2003, 51, 984–985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Negri, G.; Salatino, M.L.F.; Salatino, A. ‘Green propolis’: Unreported constituents and a novel compound from chloroform extracts. J. Apic. Res. 2003, 42, 39–41. [Google Scholar] [CrossRef]
- Trusheva, B.; Popova, M.; Bankova, V.; Tsvetkova, I.; Naydenski, C.; Sabatini, A.G. A new type of European propolis, containing bioactive labdanes. Riv. Ital. Eppos 2003, 13, 3–8. [Google Scholar]
- Chen, C.-N.; Wu, C.-L.; Lin, J.-K. Propolin C from propolis induces apoptosis through activating caspases, Bid and cytochrome c release in human melanoma cells. Biochem. Pharmacol. 2004, 67, 53–66. [Google Scholar] [CrossRef]
- Kumazawa, S.; Goto, H.; Hamasaka, T.; Fukumoto, S.; Fujimoto, T.; Nakayama, T. A new prenylated flavonoid from propolis collected in Okinawa, Japan. Biosci. Biotechnol. Biochem. 2004, 68, 260–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melliou, E.; Chinou, I. Chemical analysis and antimicrobial activity of Greek propolis. Planta Med. 2004, 70, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Trusheva, B.; Popova, M.; Naydenski, H.; Tsvetkova, I.; Rodriguez, J.G.; Bankova, V. New polyisoprenylated benzophenones from Venezuelan propolis. Fitoterapia 2004, 75, 683–689. [Google Scholar] [CrossRef]
- Awale, S.; Shrestha, S.P.; Tezuka, Y.; Ueda, J.-y.; Matsushige, K.; Kadota, S. Neoflavonoids and related constituents from Nepalese propolis and their nitric oxide production inhibitory activity. J. Nat. Prod. 2005, 68, 858–864. [Google Scholar] [CrossRef]
- Da Silva, M.D.S.S.; Citó, M.G.; Chaves, M.H.; Lopes, J.A. Triterpenóides tipo cicloartano de própolis de Teresina-PI. Química Nova 2005, 28, 801–804. [Google Scholar] [CrossRef] [Green Version]
- Hernández, I.M.; Fernandez, M.C.; Cuesta-Rubio, O.; Piccinelli, A.L.; Rastrelli, L. Polyprenylated benzophenone derivatives from Cuban propolis. J. Nat. Prod. 2005, 68, 931–934. [Google Scholar] [CrossRef] [PubMed]
- Piccinelli, A.L.; Campo Fernandez, M.; Cuesta-Rubio, O.; Marquez Hernandez, I.; De Simone, F.; Rastrelli, L. Isoflavonoids isolated from Cuban propolis. J. Agric. Food Chem. 2005, 53, 9010–9016. [Google Scholar] [CrossRef]
- Kumazawa, S.; Suzuki, S.; Ahn, M.-R.; Kamihira, M.; Udagawa, Y.; Bang, K.-S.; Nakayama, T. A new chalcone from propolis collected on Jeju Island, Korea. Food Sci. Technol. Res. 2006, 12, 67–69. [Google Scholar] [CrossRef]
- Trusheva, B.; Popova, M.; Bankova, V.; Simova, S.; Marcucci, M.C.; Miorin, P.L.; Pasin, F.d.R.; Tsvetkova, I. Bioactive constituents of Brazilian red propolis. Evid. Based Complement. Altern. Med. 2006, 3, 249–254. [Google Scholar] [CrossRef]
- Huang, W.-J.; Huang, C.-H.; Wu, C.-L.; Lin, J.-K.; Chen, Y.-W.; Lin, C.-L.; Chuang, S.-E.; Huang, C.-Y.; Chen, C.-N. Propolin G, a prenylflavanone, isolated from Taiwanese propolis, induces caspase-dependent apoptosis in brain cancer cells. J. Agric. Food Chem. 2007, 55, 7366–7376. [Google Scholar] [CrossRef]
- Kumazawa, S.; Ueda, R.; Hamasaka, T.; Fukumoto, S.; Fujimoto, T.; Nakayama, T. Antioxidant prenylated flavonoids from propolis collected in Okinawa, Japan. J. Agric. Food Chem. 2007, 55, 7722–7725. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.P.; Narukawa, Y.; Takeda, T. Chemical constituents of Nepalese propolis: Isolation of new dalbergiones and related compounds. J. Nat. Med. 2007, 61, 73–76. [Google Scholar] [CrossRef]
- Shrestha, S.P.; Narukawa, Y.; Takeda, T. Chemical constituents of Nepalese propolis (II). Chem. Pharm. Bull. 2007, 55, 926–929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freitas, M.O.; Ponte, F.A.F.; Lima, M.A.S.; Silveira, E.R. Flavonoids and triterpenes from the nest of the stingless bee Trigona spinipes. J. Braz. Chem. Soc. 2008, 19, 532–535. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Awale, S.; Tezuka, Y.; Kadota, S. Cytotoxic constituents from Brazilian red propolis and their structure–activity relationship. Bioorg. Med. Chem. 2008, 16, 5434–5440. [Google Scholar] [CrossRef]
- Silva, M.S.S.; De Lima, S.G.; Oliveira, E.H.; Lopes, J.A.D.; Chaves, M.H.; Reis, F.A.M.; Citó, A.M.G.L. Anacardic acid derivatives from Brazilian propolis and their antibacterial activity. Eclet. Quím. 2008, 33, 53–58. [Google Scholar] [CrossRef] [Green Version]
- Castro, M.L.; do Nascimento, A.M.; Ikegaki, M.; Costa-Neto, C.M.; Alencar, S.M.; Rosalen, P.L. Identification of a bioactive compound isolated from Brazilian propolis type 6. Bioorg. Med. Chem. 2009, 17, 5332–5335. [Google Scholar] [CrossRef]
- El-Bassuony, A.A. New prenilated compound from Egyptian propolis with antimicrobial activity. Rev. Latinoam. Quim. 2009, 37, 85–90. [Google Scholar]
- Li, F.; Awale, S.; Zhang, H.; Tezuka, Y.; Esumi, H.; Kadota, S. Chemical constituents of propolis from Myanmar and their preferential cytotoxicity against a human pancreatic cancer cell line. J. Nat. Prod. 2009, 72, 1283–1287. [Google Scholar] [CrossRef]
- Lima, B.; Tapia, A.; Luna, L.; Fabani, M.P.; Schmeda-Hirschmann, G.; Podio, N.S.; Wunderlin, D.A.; Feresin, G.E. Main flavonoids, DPPH activity, and metal content allow determination of the geographical origin of propolis from the province of San Juan (Argentina). J. Agric. Food Chem. 2009, 57, 2691–2698. [Google Scholar] [CrossRef]
- Meneses, E.A.; Durango, D.L.; García, C.M. Antifungal activity against postharvest fungi by extracts from Colombian propolis. Quím. Nova 2009, 32, 2011–2017. [Google Scholar] [CrossRef] [Green Version]
- Popova, M.P.; Chinou, I.B.; Marekov, I.N.; Bankova, V.S. Terpenes with antimicrobial activity from Cretan propolis. Phytochemistry 2009, 70, 1262–1271. [Google Scholar] [CrossRef] [PubMed]
- Sha, N.; Guan, S.-H.; Lu, Z.-Q.; Chen, G.-T.; Huang, H.-L.; Xie, F.-B.; Yue, Q.-X.; Liu, X.; Guo, D.-A. Cytotoxic constituents of Chinese propolis. J. Nat. Prod. 2009, 72, 799–801. [Google Scholar] [CrossRef] [PubMed]
- Agüero, M.A.B.N.; Gonzalez, M.; Lima, B.; Svetaz, L.; Sanchez, M.; Zacchino, S.; Feresin, G.E.; Schmeda-Hirschmann, G.; Palermo, J.; Wunderlin, D. Argentinean propolis from Zuccagnia punctata Cav. (Caesalpinieae) exudates: Phytochemical characterization and antifungal activity. J. Agric. Food Chem. 2010, 58, 194–201. [Google Scholar]
- Díaz-Carballo, D.; Ueberla, K.; Kleff, V.; Ergun, S.; Malak, S.; Freistuehler, M.; Somogyi, S.; Kücherer, C.; Bardenheuer, W.; Strumberg, D. Antiretroviral activity of two polyisoprenylated acylphloroglucinols, 7-epi-nemorosone and plukenetione A, isolated from Caribbean propolis. Int. J. Clin. Pharmacol. Ther. 2010, 48, 670. [Google Scholar] [CrossRef]
- El-Bassuony, A.; AbouZid, S. A new prenylated flavanoid with antibacterial activity from propolis collected in Egypt. Nat. Prod. Commun. 2010, 5, 43–45. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Awale, S.; Tezuka, Y.; Esumi, H.; Kadota, S. Study on the constituents of Mexican propolis and their cytotoxic activity against PANC-1 human pancreatic cancer cells. J. Nat. Prod. 2010, 73, 623–627. [Google Scholar] [CrossRef]
- Li, F.; Awale, S.; Tezuka, Y.; Kadota, S. Cytotoxicity of constituents from Mexican propolis against a panel of six different cancer cell lines. Nat. Prod. Commun. 2010, 5, 1601–1606. [Google Scholar] [CrossRef] [Green Version]
- Lotti, C.; Campo Fernandez, M.; Piccinelli, A.L.; Cuesta-Rubio, O.; Marquez Hernandez, I.; Rastrelli, L. Chemical constituents of red Mexican propolis. J. Agric. Food Chem. 2010, 58, 2209–2213. [Google Scholar] [CrossRef]
- Petrova, A.; Popova, M.; Kuzmanova, C.; Tsvetkova, I.; Naydenski, H.; Muli, E.; Bankova, V. New biologically active compounds from Kenyan propolis. Fitoterapia 2010, 81, 509–514. [Google Scholar] [CrossRef]
- Pratsinis, H.; Kletsas, D.; Melliou, E.; Chinou, I. Antiproliferative activity of Greek propolis. J. Med. Food 2010, 13, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Raghukumar, R.; Vali, L.; Watson, D.; Fearnley, J.; Seidel, V. Antimethicillin-resistant Staphylococcus aureus (MRSA) activity of ‘pacific propolis’ and isolated prenylflavanones. Phytother. Res. 2010, 24, 1181–1187. [Google Scholar] [PubMed]
- Trusheva, B.; Todorov, I.; Ninova, M.; Najdenski, H.; Daneshmand, A.; Bankova, V. Antibacterial mono-and sesquiterpene esters of benzoic acids from Iranian propolis. Chem. Cent. J. 2010, 4, 8. [Google Scholar] [CrossRef] [Green Version]
- Agüero, M.B.; Svetaz, L.; Sánchez, M.; Luna, L.; Lima, B.; López, M.L.; Zacchino, S.; Palermo, J.; Wunderlin, D.; Feresin, G.E. Argentinean Andean propolis associated with the medicinal plant Larrea nitida Cav. (Zygophyllaceae). HPLC–MS and GC–MS characterization and antifungal activity. Food Chem. Toxicol. 2011, 49, 1970–1978. [Google Scholar]
- Li, F.; He, Y.-M.; Awale, S.; Kadota, S.; Tezuka, Y. Two new cytotoxic phenylallylflavanones from Mexican propolis. Chem. Pharm. Bull. 2011, 59, 1194–1196. [Google Scholar] [CrossRef] [Green Version]
- Piccinelli, A.L.; Lotti, C.; Campone, L.; Cuesta-Rubio, O.; Campo Fernandez, M.; Rastrelli, L. Cuban and Brazilian red propolis: Botanical origin and comparative analysis by high-performance liquid chromatography–photodiode array detection/electrospray ionization tandem mass spectrometry. J. Agric. Food Chem. 2011, 59, 6484–6491. [Google Scholar] [CrossRef]
- Popova, M.; Trusheva, B.; Antonova, D.; Cutajar, S.; Mifsud, D.; Farrugia, C.; Tsvetkova, I.; Najdenski, H.; Bankova, V. The specific chemical profile of Mediterranean propolis from Malta. Food Chem. 2011, 126, 1431–1435. [Google Scholar] [CrossRef] [Green Version]
- Segueni, N.; Magid, A.A.; Decarme, M.; Rhouati, S.; Lahouel, M.; Antonicelli, F.; Lavaud, C.; Hornebeck, W. Inhibition of stromelysin-1 by caffeic acid derivatives from a propolis sample from Algeria. Planta Med. 2011, 77, 999–1004. [Google Scholar] [CrossRef] [Green Version]
- Shaheen, S.A.; Zarga, M.H.A.; Nazer, I.K.; Darwish, R.M.; Al-Jaber, H.I. Chemical constituents of Jordanian propolis. Nat. Prod. Res. 2011, 25, 1312–1318. [Google Scholar] [CrossRef]
- Trusheva, B.; Popova, M.; Koendhori, E.B.; Tsvetkova, I.; Naydenski, C.; Bankova, V. Indonesian propolis: Chemical composition, biological activity and botanical origin. Nat. Prod. Res. 2011, 25, 606–613. [Google Scholar] [CrossRef]
- Vera, N.; Solorzano, E.; Ordoñez, R.; Maldonado, L.; Bedascarrasbure, E.; Isla, M.I. Chemical composition of Argentinean propolis collected in extreme regions and its relation with antimicrobial and antioxidant activities. Nat. Prod. Commun. 2011, 6, 823–827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abu-Mellal, A.; Koolaji, N.; Duke, R.K.; Tran, V.H.; Duke, C.C. Prenylated cinnamate and stilbenes from Kangaroo island propolis and their antioxidant activity. Phytochemistry 2012, 77, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Inui, S.; Hosoya, T.; Shimamura, Y.; Masuda, S.; Ogawa, T.; Kobayashi, H.; Shirafuji, K.; Moli, R.T.; Kozone, I.; Shin-ya, K. Solophenols B–D and solomonin: New prenylated polyphenols isolated from propolis collected from the Solomon Islands and their antibacterial activity. J. Agric. Food Chem. 2012, 60, 11765–11770. [Google Scholar] [CrossRef] [PubMed]
- Inui, S.; Shimamura, Y.; Masuda, S.; Shirafuji, K.; Moli, R.T.; Kumazawa, S. A new prenylflavonoid isolated from propolis collected in the Solomon Islands. Biosci. Biotechnol. Biochem. 2012, 76, 1038–1040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lotti, C.; Piccinelli, A.L.; Arevalo, C.; Ruiz, I.; Migliani De Castro, G.M.; Figueira Reis De Sá, L.; Tessis, A.C.; Ferreira-Pereira, A.; Rastrelli, L. Constituents of Hondurian propolis with inhibitory effects on Saccharomyces cerevisiae multidrug resistance protein Pdr5p. J. Agric. Food Chem. 2012, 60, 10540–10545. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Yang, H.; Zhang, X.; Sheng, Y.; Huang, H.; Yu, L. Isolation and characterization of five glycerol esters from Wuhan propolis and their potential anti-inflammatory properties. J. Agric. Food Chem. 2012, 60, 10041–10047. [Google Scholar] [CrossRef] [PubMed]
- Tran, V.H.; Duke, R.K.; Abu-Mellal, A.; Duke, C.C. Propolis with high flavonoid content collected by honey bees from Acacia paradoxa. Phytochemistry 2012, 81, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Alves de Souza, S.; Camara, C.A.; Monica Sarmento Da Silva, E.; Silva, T.M.S. Composition and antioxidant activity of geopropolis collected by Melipona subnitida (Jandaíra) bees. Evid. Based Complement. Altern. Med. 2013, 801383. [Google Scholar] [CrossRef] [Green Version]
- Athikomkulchai, S.; Awale, S.; Ruangrungsi, N.; Ruchirawat, S.; Kadota, S. Chemical constituents of Thai propolis. Fitoterapia 2013, 88, 96–100. [Google Scholar] [CrossRef]
- Piccinelli, A.L.; Mencherini, T.; Celano, R.; Mouhoubi, Z.; Tamendjari, A.; Aquino, R.P.; Rastrelli, L. Chemical composition and antioxidant activity of Algerian propolis. J. Agric. Food Chem. 2013, 61, 5080–5088. [Google Scholar] [CrossRef]
- Popova, M.; Dimitrova, R.; Al-Lawati, H.T.; Tsvetkova, I.; Najdenski, H.; Bankova, V. Omani propolis: Chemical profiling, antibacterial activity and new propolis plant sources. Chem. Cent. J. 2013, 7, 158. [Google Scholar] [CrossRef] [Green Version]
- Silva, E.C.C.D.; Muniz, M.P.; Nunomura, R.D.C.S.; Nunomura, S.M.; Zilse, G.A.C. Constituintes fenólicos e atividade antioxidante da geoprópolis de duas espécies de abelhas sem ferrão amazônicas. Quím. Nova 2013, 36, 628–633. [Google Scholar] [CrossRef] [Green Version]
- Almutairi, S.; Eapen, B.; Chundi, S.M.; Akhalil, A.; Siheri, W.; Clements, C.; Fearnley, J.; Watson, D.G.; Edrada-Ebel, R. New anti-trypanosomal active prenylated compounds from African propolis. Phytochem. Lett. 2014, 10, 35–39. [Google Scholar] [CrossRef]
- Almutairi, S.; Edrada-Ebel, R.; Fearnley, J.; Igoli, J.O.; Alotaibi, W.; Clements, C.J.; Gray, A.I.; Watson, D.G. Isolation of diterpenes and flavonoids from a new type of propolis from Saudi Arabia. Phytochem. Lett. 2014, 10, 160–163. [Google Scholar] [CrossRef]
- Díaz-Carballo, D.; Acikelli, A.H.; Bardenheuer, W.; Gustmann, S.; Malak, S.; Stoll, R.; Kedziorski, T.; Nazif, M.A.; Jastrow, H.; Wennemuth, G. Identification of compounds that selectively target highly chemotherapy refractory neuroblastoma cancer stem cells. Int. J. Clin. Pharmacol. Ther. 2014, 52, 787–801. [Google Scholar] [CrossRef] [PubMed]
- Kardar, M.N.; Zhang, T.; Coxon, G.D.; Watson, D.G.; Fearnley, J.; Seidel, V. Characterisation of triterpenes and new phenolic lipids in Cameroonian propolis. Phytochemistry 2014, 106, 156–163. [Google Scholar] [CrossRef] [Green Version]
- Massaro, C.F.; Katouli, M.; Grkovic, T.; Vu, H.; Quinn, R.J.; Heard, T.A.; Carvalho, C.; Manley-Harris, M.; Wallace, H.; Brooks, P. Anti-staphylococcal activity of C-methyl flavanones from propolis of Australian stingless bees (Tetragonula carbonaria) and fruit resins of Corymbia torelliana (Myrtaceae). Fitoterapia 2014, 95, 247–257. [Google Scholar] [CrossRef]
- Paul, S.; Emmanuel, T.; Matchawe, C.; Alembert, T.T.; Sophie, L.; Maurice, T.F.; Joel, Y.G.A.; De, T.A. Pentacyclic triterpenes and crude extracts with antimicrobial activity from Cameroonian brown propolis samples. J. Appl. Pharm. Sci. 2014, 4, 1. [Google Scholar]
- Siheri, W.; Igoli, J.O.; Gray, A.I.; Nasciemento, T.G.; Zhang, T.; Fearnley, J.; Clements, C.J.; Carter, K.C.; Carruthers, J.; Edrada-Ebel, R. The isolation of antiprotozoal compounds from Libyan propolis. Phytother. Res. 2014, 28, 1756–1760. [Google Scholar] [CrossRef] [Green Version]
- Alday, E.; Valencia, D.; Carreño, A.L.; Picerno, P.; Piccinelli, A.L.; Rastrelli, L.; Robles-Zepeda, R.; Hernandez, J.; Velazquez, C. Apoptotic induction by pinobanksin and some of its ester derivatives from Sonoran propolis in a B-cell lymphoma cell line. Chem. Biol. Interact. 2015, 242, 35–44. [Google Scholar] [CrossRef]
- Funakoshi-Tago, M.; Okamoto, K.; Izumi, R.; Tago, K.; Yanagisawa, K.; Narukawa, Y.; Kiuchi, F.; Kasahara, T.; Tamura, H. Anti-inflammatory activity of flavonoids in Nepalese propolis is attributed to inhibition of the IL-33 signaling pathway. Int. Immunopharmacol. 2015, 25, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Kustiawan, P.M.; Phuwapraisirisan, P.; Puthong, S.; Palaga, T.; Arung, E.T.; Chanchao, C. Propolis from the stingless bee Trigona incisa from East Kalimantan, Indonesia, induces in vitro cytotoxicity and apoptosis in cancer cell lines. Asian Pac. J. Cancer Prev. 2015, 16, 6581–6589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ragasa, C.Y.; Galian, R.A.F.; Ebajo, V.D., Jr.; Aguda, R.M.; Cervancia, C.R.; Shen, C.-C. Propolins and glyasperin A from stingless bee nests. Rev. Bras. Farmacogn. 2015, 25, 177–179. [Google Scholar] [CrossRef] [Green Version]
- Sanpa, S.; Popova, M.; Bankova, V.; Tunkasiri, T.; Eitssayeam, S.; Chantawannakul, P. Antibacterial compounds from propolis of Tetragonula laeviceps and Tetrigona melanoleuca (Hymenoptera: Apidae) from Thailand. PLoS ONE 2015, 10, e0126886. [Google Scholar] [CrossRef] [PubMed]
- Da Cunha, M.G.; Rosalen, P.L.; Franchin, M.; De Alencar, S.M.; Ikegaki, M.; Ransom, T.; Beutler, J.A. Antiproliferative constituents of geopropolis from the bee Melipona scutellaris. Planta Med. 2016, 82, 190–194. [Google Scholar] [CrossRef] [Green Version]
- Nishimura, E.; Murakami, S.; Suzuki, K.; Amano, K.; Tanaka, R.; Shinada, T. Structure determination of monomeric phloroglucinol derivatives with a cinnamoyl group isolated from propolis of the stingless bee, Tetragonula carbonaria. Asian J. Org. Chem. 2016, 5, 855–859. [Google Scholar] [CrossRef]
- Omar, R.M.; Igoli, J.; Gray, A.I.; Ebiloma, G.U.; Clements, C.; Fearnley, J.; Edrada Ebel, R.A.; Zhang, T.; De Koning, H.P.; Watson, D.G. Chemical characterisation of Nigerian red propolis and its biological activity against Trypanosoma brucei. Phytochem. Anal. 2016, 27, 107–115. [Google Scholar] [CrossRef] [Green Version]
- Segueni, N.; Zellagui, A.; Moussaoui, F.; Lahouel, M.; Rhouati, S. Flavonoids from Algerian propolis. Arab. J. Chem. 2016, 9, S425–S428. [Google Scholar] [CrossRef] [Green Version]
- Trusheva, B.; Stancheva, K.; Gajbhiye, N.; Dimitrova, R.; Popova, M.; Saraf, R.; Bankova, V. Two new prenylated stilbenes with an irregular sesquiterpenyl side chain from propolis from Fiji Islands. Rec. Nat. Prod. 2016, 10, 465–471. [Google Scholar]
- Aminimoghadamfarouj, N.; Nematollahi, A. Structure elucidation and botanical characterization of diterpenes from a specific type of bee glue. Molecules 2017, 22, 1185. [Google Scholar] [CrossRef] [Green Version]
- Duke, C.C.; Tran, V.H.; Duke, R.K.; Abu-Mellal, A.; Plunkett, G.T.; King, D.I.; Hamid, K.; Wilson, K.L.; Barrett, R.L.; Bruhl, J.J. A sedge plant as the source of Kangaroo Island propolis rich in prenylated p-coumarate ester and stilbenes. Phytochemistry 2017, 134, 87–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, H.X.; Nguyen, M.T.; Nguyen, N.T.; Awale, S. Chemical constituents of propolis from Vietnamese trigona minor and their antiausterity activity against the panc-1 human pancreatic cancer cell line. J. Nat. Prod. 2017, 80, 2345–2352. [Google Scholar] [CrossRef] [PubMed]
- Omar, R.; Igoli, J.O.; Zhang, T.; Gray, A.I.; Ebiloma, G.U.; Clements, C.J.; Fearnley, J.; Ebel, R.E.; Paget, T.; De Koning, H.P. The chemical characterization of Nigerian propolis samples and their activity against Trypanosoma brucei. Sci. Rep. 2017, 7, 923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanpa, S.; Popova, M.; Tunkasiri, T.; Eitssayeam, S.; Bankova, V.; Chantawannakul, P. Chemical profiles and antimicrobial activities of Thai propolis collected from Apis mellifera. Chiang Mai J. Sci. 2017, 44, 438–448. [Google Scholar]
- Zhao, L.; Yu, M.; Sun, M.; Xue, X.; Wang, T.; Cao, W.; Sun, L. Rapid determination of major compounds in the ethanol extract of geopropolis from Malaysian stingless bees, Heterotrigona itama, by UHPLC-Q-TOF/MS and NMR. Molecules 2017, 22, 1935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guzmán-Gutiérrez, S.; Nieto-Camacho, A.; Castillo-Arellano, J.; Huerta-Salazar, E.; Hernández-Pasteur, G.; Silva-Miranda, M.; Argüello-Nájera, O.; Sepúlveda-Robles, O.; Espitia, C.; Reyes-Chilpa, R. Mexican propolis: A source of antioxidants and anti-inflammatory compounds, and isolation of a novel chalcone and ε-caprolactone derivative. Molecules 2018, 23, 334. [Google Scholar] [CrossRef] [Green Version]
- Ishizu, E.; Honda, S.; Vongsak, B.; Kumazawa, S. Identification of plant origin of propolis from Thailand stingless bees by comparative analysis. Nat. Prod. Commun. 2018, 13, 973–975. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, H.X.; Van Do, T.N.; Nguyen, M.T.T.; Dang, P.H.; Tho, L.H.; Awale, S.; Nguyen, N.T. A new alkenylphenol from the propolis of stingless bee Trigona minor. Nat. Prod. Commun. 2018, 13, 69–70. [Google Scholar] [CrossRef]
- Thanh, L.N.; Oanh, V.T.K.; Thoa, H.T.; Phuong, D.T.L.; Lien, N.T.P.; Giap, T.H.; Hang, N.T.M.; Hung, N.V.; Bankova, V. Isolated triterpenes from stingless bee Lisotrigona furva propolis in Vietnam. J. Apither. Nat. 2018, 1, 73. [Google Scholar]
- Alaribe, C.S.; Esposito, T.; Sansone, F.; Sunday, A.; Pagano, I.; Piccinelli, A.L.; Celano, R.; Cuesta Rubio, O.; Coker, H.A.; Nabavi, S.M. Nigerian propolis: Chemical composition, antioxidant activity and α-amylase and α-glucosidase inhibition. Nat. Prod. Res. 2019, 1–5. [Google Scholar] [CrossRef]
- Bloor, S.; Catchpole, O.; Mitchell, K.; Webby, R.; Davis, P. Antiproliferative acylated glycerols from New Zealand Propolis. J. Nat. Prod. 2019, 82, 2359–2367. [Google Scholar] [CrossRef]
- Bouaroura, A.; Segueni, N.; Diaz, J.G.; Bensouici, C.; Akkal, S.; Rhouati, S. Preliminary analysis of the chemical composition, antioxidant and anticholinesterase activities of Algerian propolis. Nat. Prod. Res. 2019, 1–5. [Google Scholar] [CrossRef]
- Georgieva, K.; Popova, M.; Dimitrova, L.; Trusheva, B.; Phuong, D.T.L.; Lien, N.T.P.; Najdenski, H.; Bankova, V. Phytochemical analysis of Vietnamese propolis produced by the stingless bee Lisotrigona cacciae. PLoS ONE 2019, 14, e0216074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrera-López, M.G.; Rubio-Hernández, E.I.; Leyte-Lugo, M.A.; Schinkovitz, A.; Richomme, P.; Calvo-Irabién, L.M.; Peña-Rodríguez, L.M. Botanical origin of triterpenoids from Yucatecan propolis. Phytochem. Lett. 2019, 29, 25–29. [Google Scholar] [CrossRef]
- Popova, M.P.; Trusheva, B.S.; Nedialkov, P.T.; Tsvetkova, I.; Pardo-Mora, D.P.; Najdenski, H.; Torres-García, O.A.; Sforcin, J.M.; Bankova, V.S. New Δ-tocotrienol derivatives from Colombian propolis. Nat. Prod. Res. 2019, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pujirahayu, N.; Suzuki, T.; Katayama, T. Cycloartane-type triterpenes and botanical origin of propolis of stingless Indonesian bee Tetragonula sapiens. Plants 2019, 8, 57. [Google Scholar] [CrossRef] [Green Version]
- Siheri, W.; Ebiloma, G.U.; Igoli, J.O.; Gray, A.I.; Biddau, M.; Akrachalanont, P.; Alenezi, S.; Alwashih, M.A.; Edrada-Ebel, R.; Muller, S. Isolation of a novel flavanonol and an alkylresorcinol with highly potent anti-trypanosomal activity from Libyan propolis. Molecules 2019, 24, 1041. [Google Scholar] [CrossRef] [Green Version]
- Tani, H.; Hikami, S.; Takahashi, S.; Kimura, Y.; Matsuura, N.; Nakamura, T.; Yamaga, M.; Koshino, H. Isolation, identification, and synthesis of a new prenylated cinnamic acid derivative from Brazilian green propolis and simultaneous quantification of bioactive components by LC-MS/MS. J. Agric. Food Chem. 2019, 67, 12303–12312. [Google Scholar] [CrossRef]
- Muli, E.; Maingi, J.M.; Macharia, J. Antimicrobial properties of propolis and honey from the Kenyan stingless bee, Dactylurina schimidti. Apiacta 2008, 43, 49–61. [Google Scholar]
- Anibijuwon, I.; Gbala, I.; Adeyemi, J.; Abioye, J. Antibacterial activity of stingless bee (Dactylurina studingeri) propolis on bacteria isolated from wound. SMU Med. J. 2017, 4, 43–50. [Google Scholar]
- Bankova, V. Chemical diversity of propolis and the problem of standardization. J. Ethnopharmacol. 2005, 100, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Bankova, V.; Popova, M. Propolis of stingless bees: A promising source of biologically active compounds. Pharmacogn. Rev. 2007, 1, 88–92. [Google Scholar]
- Shen, X.; Liu, Y.; Luo, X.; Yang, Z. Advances in biosynthesis, pharmacology, and pharmacokinetics of pinocembrin, a promising natural small-molecule drug. Molecules 2019, 24, 2323. [Google Scholar] [CrossRef] [Green Version]
- Mani, R.; Natesan, V. Chrysin: Sources, beneficial pharmacological activities, and molecular mechanism of action. Phytochemistry 2018, 145, 187–196. [Google Scholar] [CrossRef]
- Murtaza, G.; Karim, S.; Akram, M.R.; Khan, S.A.; Azhar, S.; Mumtaz, A.; Asad, B.; Hassan, M.H. Caffeic acid phenethyl ester and therapeutic potentials. Biomed. Res. Int. 2014, 2014, 145342. [Google Scholar] [CrossRef] [Green Version]
- Su, K.-Y.; Hsieh, C.-Y.; Chen, Y.-W.; Chuang, C.-T.; Chen, C.-T.; Chen, Y.-L.S. Taiwanese green propolis and propolin G protect the liver from the pathogenesis of fibrosis via eliminating TGF-β-induced smad2/3 phosphorylation. J. Agric. Food Chem. 2014, 62, 3192–3201. [Google Scholar] [CrossRef] [PubMed]
- Bueno-Silva, B.; Alencar, S.M.; Koo, H.; Ikegaki, M.; Silva, G.V.J.; Napimoga, M.H.; Rosalen, P.L. Anti-inflammatory and antimicrobial evaluation of neovestitol and vestitol isolated from Brazilian red propolis. J. Agric. Food Chem. 2013, 61, 4546–4550. [Google Scholar] [CrossRef]
- Promchai, T.; Janhom, P.; Maneerat, W.; Rattanajak, R.; Kamchonwongpaisan, S.; Pyne, S.G.; Limtharakul, T. Antibacterial and cytotoxic activities of phenolic constituents from the stem extracts of Spatholobus parviflorus. Nat. Prod. Res. 2020, 34, 1394–1398. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, Y.; Hsieh, M.-T.; Xin, G.; Wu, R.-T.; Hsu, P.-L.; Horng, L.-Y.; Sung, H.-C.; Cheng, C.-H.; Lee, K.-H. Total synthesis of (+)-medicarpin. J. Nat. Prod. 2017, 80, 3284–3288. [Google Scholar] [CrossRef]
- Dixit, M.; Raghuvanshi, A.; Gupta, C.P.; Kureel, J.; Mansoori, M.N.; Shukla, P.; John, A.A.; Singh, K.; Purohit, D.; Awasthi, P. Medicarpin, a natural pterocarpan, heals cortical bone defect by activation of notch and wnt canonical signaling pathways. PLoS ONE 2015, 10, e0144541. [Google Scholar] [CrossRef]
- Oldoni, T.L.C.; Cabral, I.S.R.; D’Arce, M.A.B.R.; Rosalen, P.L.; Ikegaki, M.; Nascimento, A.M.; Alencar, S.M. Isolation and analysis of bioactive isoflavonoids and chalcone from a new type of Brazilian propolis. Sep. Purif. Technol. 2011, 77, 208–213. [Google Scholar] [CrossRef]
- Franchin, M.; Cólon, D.F.; Castanheira, F.V.S.; da Cunha, M.G.; Bueno-Silva, B.; Alencar, S.M.; Cunha, T.M.; Rosalen, P.L. Vestitol isolated from Brazilian red propolis inhibits neutrophils migration in the inflammatory process: Elucidation of the mechanism of action. J. Nat. Prod. 2016, 79, 954–960. [Google Scholar] [CrossRef]
- Duque Estrada, G.O.; Mendes da Silva, J.F.; Ceva Antunes, O.A. Artepillin C: A review. Lett. Drug Des. Discov. 2008, 5, 88–92. [Google Scholar]
- Paulino, N.; Abreu, S.R.L.; Uto, Y.; Koyama, D.; Nagasawa, H.; Hori, H.; Dirsch, V.M.; Vollmar, A.M.; Scremin, A.; Bretz, W.A. Anti-inflammatory effects of a bioavailable compound, Artepillin C, in Brazilian propolis. Eur. J. Pharmacol. 2008, 587, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Sparling, B.A.; Tucker, J.K.; Moebius, D.C.; Shair, M.D. Total synthesis of (−)-nemorosone and (+)-secohyperforin. Org. Lett. 2015, 17, 3398–3401. [Google Scholar] [CrossRef] [PubMed]
- Ninh The, S. A review on the medicinal plant Dalbergia odorifera species: Phytochemistry and biological activity. Evid. Based Complement. Altern. Med. 2017, 2017, 7142370. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Li, Y.; Wang, W.; Deng, L. Bioactivity and pharmacological properties of α-mangostin from the mangosteen fruit: A review. Expert Opin. Ther. Pat. 2018, 28, 415–427. [Google Scholar] [CrossRef]
- Franchin, M.; Rosalen, P.L.; da Cunha, M.G.; Silva, R.L.; Colon, D.F.; Bassi, G.S.; de Alencar, S.M.; Ikegaki, M.; Alves-Filho, J.C.; Cunha, F.Q. Cinnamoyloxy-mammeisin isolated from geopropolis attenuates inflammatory process by inhibiting cytokine production: Involvement of MAPK, AP-1, and NF-κB. J. Nat. Prod. 2016, 79, 1828–1833. [Google Scholar] [CrossRef]
- Da Cunha, M.G.; Sardi, J.D.C.O.; Freires, I.A.; Franchin, M.; Rosalen, P.L. Antimicrobial, anti-adherence and antibiofilm activity against Staphylococcus aureus of a 4-phenyl coumarin derivative isolated from Brazilian geopropolis. Microb. Pathog. 2020, 139, 103855. [Google Scholar] [CrossRef]
- Marcucci, M.C.; Ferreres, F.; Garc;ía-Viguera, C.; Bankova, V.S.; De Castro, S.L.; Dantas, A.P.; Valente, P.H.M.; Paulino, N. Phenolic compounds from Brazilian propolis with pharmacological activities. J. Ethnopharmacol. 2001, 74, 105–112. [Google Scholar] [CrossRef]
- Uto, Y.; Ae, S.; Koyama, D.; Sakakibara, M.; Otomo, N.; Otsuki, M.; Nagasawa, H.; Kirk, K.L.; Hori, H. Artepillin C isoprenomics: Design and synthesis of artepillin C isoprene analogues as lipid peroxidation inhibitor having low mitochondrial toxicity. Bioorg. Med. Chem. 2006, 14, 5721–5728. [Google Scholar] [CrossRef] [PubMed]
- Kimoto, T.; Aga, M.; Hino, K.; Koya-Miyata, S.; Yamamoto, Y.; Micallef, M.J.; Hanaya, T.; Arai, S.; Ikeda, M.; Kurimoto, M. Apoptosis of human leukemia cells induced by artepillin C, an active ingredient of Brazilian propolis. Anticancer Res. 2001, 21, 221–228. [Google Scholar]
- Akao, Y.; Maruyama, H.; Matsumoto, K.; Ohguchi, K.; Nishizawa, K.; Sakamoto, T.; Araki, Y.; Mishima, S.; Nozawa, Y. Cell growth inhibitory effect of cinnamic acid derivatives from propolis on human tumor cell lines. Biol. Pharm. Bull. 2003, 26, 1057–1059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahn, M.-R.; Kunimasa, K.; Ohta, T.; Kumazawa, S.; Kamihira, M.; Kaji, K.; Uto, Y.; Hori, H.; Nagasawa, H.; Nakayama, T. Suppression of tumor-induced angiogenesis by Brazilian propolis: Major component artepillin C inhibits in vitro tube formation and endothelial cell proliferation. Cancer Lett. 2007, 252, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, N.; Kakino, Y.; Iwai, K.; Mochida, K.Y.O.; Fujita, T. In vitro antibacterial, antimutagenic and anti-Influenza virus activity of caffeic acid phenethyl esters. Biocontrol Sci. 2005, 10, 155–161. [Google Scholar] [CrossRef] [Green Version]
- Shen, H.; Yamashita, A.; Nakakoshi, M.; Yokoe, H.; Sudo, M.; Kasai, H.; Tanaka, T.; Fujimoto, Y.; Ikeda, M.; Kato, N. Inhibitory effects of caffeic acid phenethyl ester derivatives on replication of hepatitis C virus. PLoS ONE 2013, 8, e82299. [Google Scholar] [CrossRef]
- Sud’ina, G.F.; Mirzoeva, O.K.; Pushkareva, M.A.; Korshunova, G.A.; Sumbatyan, N.V.; Varfolomeev, S.D. Caffeic acid phenethyl ester as a lipoxygenase inhibitor with antioxidant properties. FEBS Lett. 1993, 329, 21–24. [Google Scholar] [CrossRef] [Green Version]
- Burke, T.R., Jr.; Fesen, M.; Mazumder, A.; Yung, J.; Wang, J.; Carothers, A.M.; Grunberger, D.; Driscoll, J.; Pommier, Y.; Kohn, K. Hydroxylated aromatic inhibitors of HIV-1 integrase. J. Med. Chem. 1995, 38, 4171–4178. [Google Scholar] [CrossRef]
- Michaluart, P.; Masferrer, J.L.; Carothers, A.M.; Subbaramaiah, K.; Zweifel, B.S.; Koboldt, C.; Mestre, J.R.; Grunberger, D.; Sacks, P.G.; Tanabe, T.; et al. Inhibitory effects of caffeic acid phenethyl ester on the activity and expression of cyclooxygenase-2 in human oral epithelial cells and in a rat model of inflammation. Cancer Res. 1999, 59, 2347–2352. [Google Scholar]
- Natarajan, K.; Singh, S.; Burke, T.R.; Grunberger, D.; Aggarwal, B.B. Caffeic acid phenethyl ester is a potent and specific inhibitor of activation of nuclear transcription factor NF-kappa B. Proc. Natl. Acad. Sci. USA 1996, 93, 9090–9095. [Google Scholar] [CrossRef] [Green Version]
- Da Cunha, F.M.; Duma, D.; Assreuy, J.; Buzzi, F.C.; Niero, R.; Campos, M.M.; Calixto, J.B. Caffeic acid derivatives: In vitro and in vivo anti-inflammatory properties. Free Radic. Res. 2004, 38, 1241–1253. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.S.; Xue, G.Z.; Grunberger, D.; Prystowsky, J.H. Caffeic acid phenethyl ester inhibits proliferation of human keratinocytes and interferes with the EGF regulation of ornithine decarboxylase. Oncol. Res. 1995, 7, 445–452. [Google Scholar] [PubMed]
- Liao, H.-F.; Chen, Y.-Y.; Liu, J.-J.; Hsu, M.-L.; Shieh, H.-J.; Liao, H.-J.; Shieh, C.-J.; Shiao, M.-S.; Chen, Y.-J. Inhibitory effect of caffeic acid phenethyl ester on angiogenesis, tumor invasion, and metastasis. J. Agric. Food Chem. 2003, 51, 7907–7912. [Google Scholar] [CrossRef]
- Ilhan, A.; Iraz, M.; Gurel, A.; Armutcu, F.; Akyol, O. Caffeic acid phenethyl ester exerts a neuroprotective effect on CNS against pentylenetetrazol-induced seizures in mice. Neurochem. Res. 2004, 29, 2287–2292. [Google Scholar] [CrossRef]
- Lee, K.J.; Choi, J.H.; Khanal, T.; Hwang, Y.P.; Chung, Y.C.; Jeong, H.G. Protective effect of caffeic acid phenethyl ester against carbon tetrachloride-induced hepatotoxicity in mice. Toxicology 2008, 248, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Mehri, S.; Karami, H.V.; Hassani, F.V.; Hosseinzadeh, H. Chrysin reduced acrylamide-induced neurotoxicity in both in vitro and in vivo assessments. Iran. Biomed. J. 2014, 18, 101. [Google Scholar] [PubMed]
- Wang, J.; Zhang, T.; Du, J.; Cui, S.; Yang, F.; Jin, Q. Anti-enterovirus 71 effects of chrysin and its phosphate ester. PLoS ONE 2014, 9, e89668. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.-M.; Phan, T.A.; Patel, P.N.; Jaskula-Sztul, R.; Chen, H. Chrysin activates Notch1 signaling and suppresses tumor growth of anaplastic thyroid carcinoma in vitro and in vivo. Cancer 2013, 119, 774–781. [Google Scholar] [CrossRef] [Green Version]
- Yang, B.; Huang, J.; Xiang, T.; Yin, X.; Luo, X.; Huang, J.; Luo, F.; Li, H.; Li, H.; Ren, G. Chrysin inhibits metastatic potential of human triple-negative breast cancer cells by modulating matrix metalloproteinase-10, epithelial to mesenchymal transition, and PI3K/Akt signaling pathway. J. Appl. Toxicol. 2014, 34, 105–112. [Google Scholar] [CrossRef]
- Fu, B.; Xue, J.; Li, Z.; Shi, X.; Jiang, B.-H.; Fang, J. Chrysin inhibits expression of hypoxia-inducible factor-1α through reducing hypoxia-inducible factor-1α stability and inhibiting its protein synthesis. Mol. Cancer Ther. 2007, 6, 220–226. [Google Scholar] [CrossRef] [Green Version]
- Rehman, M.U.; Ali, N.; Rashid, S.; Jain, T.; Nafees, S.; Tahir, M.; Khan, A.Q.; Lateef, A.; Khan, R.; Hamiza, O.O.; et al. Alleviation of hepatic injury by chrysin in cisplatin administered rats: Probable role of oxidative and inflammatory markers. Pharmacol. Rep. 2014, 66, 1050–1059. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, G.; Szeto, S.S.W.; Chong, C.M.; Quan, Q.; Huang, C.; Cui, W.; Guo, B.; Wang, Y.; Han, Y.; et al. Examining the neuroprotective effects of protocatechuic acid and chrysin on in vitro and in vivo models of Parkinson disease. Free Radic. Biol. Med. 2015, 84, 331–343. [Google Scholar] [CrossRef]
- Rani, N.; Bharti, S.; Bhatia, J.; Nag, T.; Ray, R.; Arya, D.S.J.C.-B.I. Chrysin, a PPAR-γ agonist improves myocardial injury in diabetic rats through inhibiting AGE-RAGE mediated oxidative stress and inflammation. Chem. Biol. Interact. 2016, 250, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Pujirahayu, N.; Bhattacharjya, D.K.; Suzuki, T.; Katayama, T. α-Glucosidase inhibitory activity of cycloartane-type triterpenes isolated from Indonesian stingless bee Propolis and their structure–activity relationship. Pharmaceuticals 2019, 12, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sivaranjani, M.; Prakash, M.; Gowrishankar, S.; Rathna, J.; Pandian, S.K.; Ravi, A.V. In vitro activity of alpha-mangostin in killing and eradicating Staphylococcus epidermidis RP62A biofilms. Appl. Microbiol. Biotechnol. 2017, 101, 3349–3359. [Google Scholar] [CrossRef] [PubMed]
- Phuong, N.T.M.; Van Quang, N.; Mai, T.T.; Anh, N.V.; Kuhakarn, C.; Reutrakul, V.; Bolhuis, A. Antibiofilm activity of α-mangostin extracted from Garcinia mangostana L. against Staphylococcus aureus. Asian Pac. J. Trop. Med. 2017, 10, 1154–1160. [Google Scholar] [CrossRef] [PubMed]
- Upegui, Y.; Robledo, S.M.; Gil Romero, J.F.; Quiñones, W.; Archbold, R.; Torres, F.; Escobar, G.; Nariño, B.; Echeverri, F. In vivo antimalarial activity of α-mangostin and the new xanthone δ-mangostin. Phytother. Res. 2015, 29, 1195–1201. [Google Scholar] [CrossRef]
- Tarasuk, M.; Songprakhon, P.; Chimma, P.; Sratongno, P.; Na-Bangchang, K.; Yenchitsomanus, P.-T. Alpha-mangostin inhibits both dengue virus production and cytokine/chemokine expression. Virus Res. 2017, 240, 180–189. [Google Scholar] [CrossRef]
- Li, P.; Tian, W.; Ma, X. Alpha-mangostin inhibits intracellular fatty acid synthase and induces apoptosis in breast cancer cells. Mol. Cancer. 2014, 13, 138. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Petiwala, S.M.; Nonn, L.; Johnson, J.J. Inhibition of CHOP accentuates the apoptotic effect of α-mangostin from the mangosteen fruit (Garcinia mangostana) in 22Rv1 prostate cancer cells. Biochem. Biophys. Res. Commun. 2014, 453, 75–80. [Google Scholar] [CrossRef]
- Franceschelli, S.; Pesce, M.; Ferrone, A.; Patruno, A.; Pasqualone, L.; Carlucci, G.; Ferrone, V.; Carlucci, M.; de Lutiis, M.A.; Grilli, A.; et al. A novel biological role of α-mangostin in modulating inflammatory response through the activation of SIRT-1 signaling pathway. J. Cell. Physiol. 2016, 231, 2439–2451. [Google Scholar] [CrossRef]
- Zhao, L.X.; Wang, Y.; Liu, T.; Wang, Y.X.; Chen, H.Z.; Xu, J.R.; Qiu, Y. α-Mangostin decreases β-amyloid peptides production via modulation of amyloidogenic pathway. CNS Neurosci. Ther. 2017, 23, 526–534. [Google Scholar] [CrossRef] [Green Version]
- Taher, M.; Amiroudine, M.; Ali, M.Z.; Zakaria, T.; Syafiq, T.M.F.; Susanti, D.; Ichwan, S.J.; Kaderi, M.A.; Ahmed, Q.U.; Zakaria, Z.A. α-Mangostin improves glucose uptake and inhibits adipocytes differentiation in 3T3-L1 cells via PPARγ, GLUT4, and leptin expressions. Evid. Based Complement. Altern. Med. 2015, 740238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Sotres, C.; López-Albarrán, P.; Cruz-de-León, J.; García-Moreno, T.; Rutiaga-Quiñones, J.G.; Vázquez-Marrufo, G.; Tamariz-Mascarúa, J.; Herrera-Bucio, R. Medicarpin, an antifungal compound identified in hexane extract of Dalbergia congestiflora Pittier heartwood. Int. Biodeterior. Biodegrad. 2012, 69, 38–40. [Google Scholar] [CrossRef]
- Kureel, J.; John, A.A.; Raghuvanshi, A.; Awasthi, P.; Goel, A.; Singh, D. Identification of GRP78 as a molecular target of medicarpin in osteoblast cells by proteomics. Mol. Cell Biochem. 2016, 418, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, R.; Maurya, R.; Mishra, D.P. Medicarpin, a legume phytoalexin sensitizes myeloid leukemia cells to TRAIL-induced apoptosis through the induction of DR5 and activation of the ROS-JNK-CHOP pathway. Cell Death Dis. 2014, 5, e1465. [Google Scholar] [CrossRef] [Green Version]
- Gatouillat, G.; Magid, A.A.; Bertin, E.; El btaouri, H.; Morjani, H.; Lavaud, C.; Madoulet, C. Medicarpin and millepurpan, two flavonoids isolated from Medicago sativa, induce apoptosis and overcome multidrug resistance in leukemia P388 cells. Phytomedicine 2015, 22, 1186–1194. [Google Scholar] [CrossRef]
- Lokvam, J.; Braddock, J.F.; Reichardt, P.B.; Clausen, T.P. Two polyisoprenylated benzophenones from the trunk latex of Clusia grandiflora (Clusiaceae). Phytochemistry 2000, 55, 29–34. [Google Scholar] [CrossRef]
- Monzote, L.; Cuesta-Rubio, O.; Matheeussen, A.; Van Assche, T.; Maes, L.; Cos, P. Antimicrobial evaluation of the polyisoprenylated benzophenones nemorosone and guttiferone A. Phytother. Res. 2011, 25, 458–462. [Google Scholar] [CrossRef] [Green Version]
- Dal Piaz, F.; Tosco, A.; Eletto, D.; Piccinelli, A.L.; Moltedo, O.; Franceschelli, S.; Sbardella, G.; Remondelli, P.; Rastrelli, L.; Vesci, L.; et al. The Identification of a novel natural activator of p300 histone acetyltranferase provides new insights into the modulation mechanism of this enzyme. ChemBioChem 2010, 11, 818–827. [Google Scholar] [CrossRef]
- Soromou, L.W.; Zhang, Y.; Cui, Y.; Wei, M.; Chen, N.; Yang, X.; Huo, M.; Baldé, A.; Guan, S.; Deng, X.; et al. Subinhibitory concentrations of pinocembrin exert anti-Staphylococcus aureus activity by reducing α-toxin expression. J. Appl. Microbiol. 2013, 115, 41–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melaku, Y.; Worku, T.; Tadesse, Y.; Mekonnen, Y.; Schmidt, J.; Arnold, N.; Dagne, E. Antiplasmodial compounds from leaves of Dodonaea angustifolia. Curr. Bioact. Compd. 2017, 13, 268–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wishart, D.S.; Feunang, Y.D.; Guo, A.C.; Lo, E.J.; Marcu, A.; Grant, J.R.; Sajed, T.; Johnson, D.; Li, C.; Sayeeda, Z. DrugBank 5.0: A major update to the DrugBank database for 2018. Nucleic Acids Res. 2018, 46, D1074–D1082. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.drugbank.ca/drugs (accessed on 29 April 2020).
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.-Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]
- Lipinski, C.A. Lead-and drug-like compounds: The rule-of-five revolution. Drug Discov. Today Technol. 2004, 1, 337–341. [Google Scholar] [CrossRef]
- Camp, D.; Garavelas, A.; Campitelli, M. Analysis of Physicochemical Properties for Drugs of Natural Origin. J. Nat. Prod. 2015, 78, 1370–1382. [Google Scholar] [CrossRef] [Green Version]
- Singh, N.; Guha, R.; Giulianotti, M.A.; Pinilla, C.; Houghten, R.A.; Medina-Franco, J.L. Chemoinformatic analysis of combinatorial libraries, drugs, natural products, and molecular libraries small molecule repository. J. Chem. Inf. Model. 2009, 49, 1010–1024. [Google Scholar] [CrossRef] [Green Version]
- Koutsoukas, A.; Paricharak, S.; Galloway, W.R.J.D.; Spring, D.R.; IJzerman, A.P.; Glen, R.C.; Marcus, D.; Bender, A. How diverse are diversity assessment methods? A comparative analysis and benchmarking of molecular descriptor space. J. Chem. Inf. Model. 2014, 54, 230–242. [Google Scholar] [CrossRef]
- González-Medina, M.; Prieto-Martínez, F.D.; Owen, J.R.; Medina-Franco, J.L. Consensus diversity plots: A global diversity analysis of chemical libraries. J. Cheminform. 2016, 8, 63. [Google Scholar] [CrossRef] [Green Version]
- Naveja, J.J.; Rico-Hidalgo, M.P.; Medina-Franco, J.L. Analysis of a large food chemical database: Chemical space, diversity, and complexity. F1000Research 2018, 7, 993. [Google Scholar] [CrossRef]
- González-Medina, M.; Medina-Franco, J.L. Chemical diversity of cyanobacterial compounds: A chemoinformatics analysis. ACS Omega 2019, 4, 6229–6237. [Google Scholar] [CrossRef] [Green Version]
- Bajorath, J. Computational scaffold hopping: Cornerstone for the future of drug design? Future Med. Chem. 2017, 9, 629–631. [Google Scholar] [CrossRef] [PubMed]
- Dimova, D.; Stumpfe, D.; Hu, Y.; Bajorath, J. Analog series-based scaffolds: Computational design and exploration of a new type of molecular scaffolds for medicinal chemistry. Future Sci. OA 2016, 2, FSO149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lipkus, A.H.; Yuan, Q.; Lucas, K.A.; Funk, S.A.; Bartelt, W.F.; Schenck, R.J.; Trippe, A.J. Structural diversity of organic chemistry. A scaffold analysis of the CAS registry. J. Org. Chem. 2008, 73, 4443–4451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medina-Franco, J.L.; Martínez-Mayorga, K.; Bender, A.; Scior, T. Scaffold diversity analysis of compound data sets using an entropy-based measure. QSAR Comb. Sci. 2009, 28, 1551–1560. [Google Scholar] [CrossRef]














| Plant Species | Plant Family | Characteristic Chemical Class | Bee Species | Country |
|---|---|---|---|---|
| Acacia paradoxa | Fabaceae | Chalcone Flavanonol | A. mellifera | Australia [121] |
| Anacardium occidentale | Anacardiaceae | Cycloartane-type triterpene | A. mellifera | Brazil [90] |
| Araucaria heterophylla | Araucariaceae | Labdane-type diterpene | A. mellifera | Brazil [48] |
| Azadirachta indica | Meliaceae | Prenylated flavanone | A. mellifera | Oman [125] |
| Baccharis spp. | Asteraceae | Flavanone/Flavanonol Flavone/Flavonol Phenylpropanoid ester Prenylated phenylpropanoid Labdane-type diterpene | A. mellifera | Brazil [53,59] |
| Betula verrucosa | Betulaceae | Flavone/Flavonol | A. mellifera | Russia [25] |
| Bursera simaruba | Burseraceae | Cycloartane-type triterpene | A. mellifera | Mexico [158] |
| Cistus spp. | Cistaceae | Labdane-type diterpene | A. mellifera | Algeria [124] |
| Clusia spp. | Clusiaceae | Polyprenylated acylphloroglucinol | A. mellifera | Cuba [66] and Venezuela [77] |
| Corymbia torelliana | Myrtaceae | Flavanone/Flavanonol | T. carbonaria | Australia [131] |
| Dalbergia spp. | Fabaceae | Pterocarpan Isoflavone Isoflavane Dalbergione | A. mellifera | Brazil [89], Cuba [81,129], Mexico [103], Nepal [78,86,87], and Nigeria [141,154] |
| Garcinia mangostana | Guttiferae | Xanthone | T. laeviceps T. pagdeni L. cacciae | Thai [138,151] and Vietnamese [157] |
| Kielmeyera sp. | Calophyllaceae | Coumarin | M. scutellaris | Brazil [139] |
| Lepidosperma spp. | Cyperaceae | Stilbene | A. mellifera | Australia [121,145] |
| Liquidambar styraciflua | Altingiaceae | Flavanone Phenylpropanoid ester | A. mellifera | Honduras [119] |
| Macaranga spp. | Euphorbiaceae | Prenylated flavanone | A. mellifera | Japan [75,85], Taiwan [70,84], Fiji [143], Solomon Island [106,117,118], Egypt [92,100] and Nigeria [141] |
| Mangifera indica | Anacardiaceae | Cycloartane-type triterpene | A. mellifera Tetragonula sapiens T. minor | Brazil [79], Indonesia [114,160], Myanmar [93], Thailand [148], Vietnam [146] |
| Pinus halepensis | Pinaceae | Flavanone/Flavanonol Flavone/Flavonol | A. mellifera | Jordan [113] |
| Populus spp. | Salicaceae | Flavanone/Flavone Phenylpropanoid ester | A. mellifera | Algeria [124,154], Mexico [101], Uruguay [68], China [120], Bulgaria [45], Netherland [65] |
| Styrax spp. | Styracaceae | Flavanone/Flavanonol Flavone/Flavonol Phenylpropanoid ester | A. mellifera | Thailand [123] |
| Xanthorrhoea spp. | Xanthorrhoeaceae | Flavanone | A. mellifera | Australia [44] |
| Zuccagnia punctate | Caesalpinieae | Flavanone/Flavonol | A. mellifera | Argentina [98] |
| Compound | Chemical Class | Phenotypic Activity | Molecular Target Activity |
|---|---|---|---|
| Artepillin C | Prenylated phenylpropanoids | Antibacteria (inhibition of B. cereus, B. Subtilis, M. lysodeikticus, P. aeruginosa, E. aerogenes, M. smegmatis, S. faecalis, E. coli, C. equi, and S. aureus [177]) Antifungi (inhibition of C. albicans, C. tropicalis, C. neoformans, S. cerevisiae, A. fumigatus, A. flavus, A. niger, M.canis, M. gypseum, E. floccosum, T. rubrum, and T. mentagrophytes [177]) Antitrypanosome (inhibition of trypomastigote forms of T. cruzi [184]) Antioxidation (in vivo inhibition of lipid peroxidation [185]) Anticancer (inhibition of human cancer cell lines [186,187,188]) | Anti-inflammation (in vitro and in vivo inhibition of NO through NF-κB [178]) |
| Caffeic acid phenyl ester—CAPE (Phenethyl caffeate) | Phenylpropanoid ester | Antibacteria (inhibition of S. aureus, B. subtilis, and P. aeruginosa [189]) Antivirus (inhibition of AH1N1 [189] and hepatitis C virus [190]) | Antioxidation (inhibition of 5-lipoxygenase [191]) Antivirus (inhibition of HIV-1 integrase [192]) Anti-inflammation (in vivo inhibition of COX- 2 [193], inhibition of NF-κB [194], in vitro and in vivo scavenging of NO and modulation of iNOS expression [195]) Anticancer (inhibition of protein kinase C [196], in vitro and in vivo inhibition of MMP-2, MMP-9 and VEGF [197]) Neuroprotection (scavenging ROS [198]) Hepatoprotection (in vivo inhibition of CYP2E1 [199]) |
| Chrysin | Flavone | Neuroprotection (in vitro and in vivo inhibition of acrylamide-induced toxicity [200]) Antivirus (inhibition of enterovirus 71 [201]) | Anticancer (in vitro and in vivo activation of Notch1 signalling [202], regulating MMP-10 and epithelial-mesenchymal transition [203], inhibition of HIF-1a [204]) Anti-inflammation (in vivo inhibition of COX-2 and iNOS [205]) Neuroprotection (inhibition of NF-κB and iNOS [206]) Antidiabetes (inhibition of AGE-RAGE mediated oxidative stress and inflammation [207]) |
| Cinnamoyloxy-mammeisin | Coumarin | Antibacteria (inhibition of methicillin-resistant S. aureus adherence to host cells and disruption of biofilm development [183]) Toxicity (low acute toxicity on Gallleria mellonella larvae model [183]) | Anti-inflammation (in vivo reduction of neutrophil migration by inhibiting the release of TNF-α and CXCL2/MIP-2 associated with inhibition of ERK 1/2, JNK, and p38 MAPK phosphorylation, AP-1, and NF-κB [182]) |
| 5,4′-Dihydroxy-3,3′-dimethoxy-2-prenyl-(E)-stilbene | Stilbene | Antioxidation (scavenging DPPH radical [116]) Anticancer (inhibiting the growth of NCI-60 cancer cell lines growth [145]) | |
| Isocupressic acid | Diterpene | Antibacteria (inhibition of S. aureus [48,73]) Antitrypanosome (inhibition of T. brucei [161]) | |
| Mangiferonic acid | Triterpene | Antitrypanosome (inhibition of T. brucei [147,161]) Antimalaria (inhibition of P. falciparum [161]) | Antidiabetes (in vitro inhibition of α-glucosidase [208]) |
| α-Mangostin | Xanthone | Antibacteria (inhibition of S. epidermidis [209], and S. aureus biofilm formation [210]) Antimalaria (inhibition of P. falciparum [211]) Antivirus (inhibition of severe dengue virus [212]) | Anticancer (inhibition of fatty acid synthase [213], PERK [214]) Anti-inflammation (inhibition of p65 acetylation, COX-2 and iNOS [215]) Neuroprotection (inhibition of self-induced β-amyloid aggregation [216]) Anti-obesity (inhibition of PPARγ [217]) |
| Medicarpin | Pterocarpan | Antibacteria (inhibition of P. aeruginosa and B. cereus [172]) Antifungi (inhibition of T. versicolor [218]) | Bone healing (in vivo bone generation by activating Wnt and notch signalling in pre-osteoblasts [174], in vitro downregulation of GRP78 [219]) Anticancer (Sensitizing human myeloid leukemia cells to TRAIL-induced apoptosis [220], enhancing cytotoxicity of chemotherapy drugs by modulating P-gp-mediated efflux [221]) |
| (S)-4-Methoxydalbergione | Dalbergione (Neoflavonoid) | Anti-inflammation (inhibition of the release of β-glucuronidase and superoxide formation induced by phorbol myristate acetate [180]) Anticancer (in vitro and in vivo suppression of osteosarcoma cells through downregulation of JAK2/STAT3 pathway [180]) | |
| Nemorosone | Polyprenylated acylphloroglucinol | Antioxidation (scavenging DPPH radical [66]) Anticancer (inhibition of cancer cell lines [66]) Antibacteria (inhibition of P. larvae, P. alvei and S. aureus [222,223]) Antimalaria (inhibition of P. falciparum [223]) Antitrypanosome (inhibition of T. brucei and T. cruzi [223]) Antileishmania (inhibition of L. amazonensis and L. infantum [223]) | Anticancer (activation of p300 histone acetyltransferase [224]) |
| Pinocembrin | Flavanone | Antibacteria (inhibition of S. aureus [225]) Antimalaria (inhibition of P. berghei [226]) | Neuroprotection (inhibition of MAPK, IκB, NF-κB p65 [167]) Anti-inflammation (inhibition of Th2 cytokines, IL-4, IL-5, IL-13, IκBα, NF-κB p65 phosphorylation, MMP-1, MMP-3, and MMP-13 [167]) Hepatoprotection (inhibition of ROS, PI3K/Akt and SMAD [167]) |
| Propolin G | Prenylated flavanone | Antioxidation (scavenging DPPH radical) [84] | Hepatoprotection (disruption of TGF-β-Smad2/3 signalling by reducing Smad2/3 formation) [170] Neuroprotection (prevention of neuronal death against oxidative stress challenges) [84] |
| Vestitol | Isoflavane | Antibacteria (inhibition of S. aureus, S. mutans, S. sobrinus and A. naeslundii growth) [171,175] Anti-inflammation (in vivo inhibition of neutrophil migration) [171] |
| Dataset | Initial Compounds | Unique Compounds b | Source |
|---|---|---|---|
| HBP | 502 a | 471 | This review |
| SBP | 100 a | 94 | This review |
| FC | 28,771 | 18,556 | http://foodb.ca/ |
| DB | 2413 | 2077 | https://www.drugbank.ca/ |
| Dataset | Size | Chemotype | Median Similarity | Scaffold Diversity (AUC) | Scaffold Diversity (F50) |
|---|---|---|---|---|---|
| HBP | 471 | 115 | 0.479 | 0.809 | 0.078 |
| SBP | 94 | 38 | 0.545 | 0.737 | 0.158 |
| FC | 3772 | 0.323 | 0.878 | 0.004 | |
| DB | 2077 | 1164 | 0.302 | 0.707 | 0.144 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, T.D.; Ogbourne, S.M.; Brooks, P.R.; Sánchez-Cruz, N.; Medina-Franco, J.L.; Quinn, R.J. Lessons from Exploring Chemical Space and Chemical Diversity of Propolis Components. Int. J. Mol. Sci. 2020, 21, 4988. https://doi.org/10.3390/ijms21144988
Tran TD, Ogbourne SM, Brooks PR, Sánchez-Cruz N, Medina-Franco JL, Quinn RJ. Lessons from Exploring Chemical Space and Chemical Diversity of Propolis Components. International Journal of Molecular Sciences. 2020; 21(14):4988. https://doi.org/10.3390/ijms21144988
Chicago/Turabian StyleTran, Trong D., Steven M. Ogbourne, Peter R. Brooks, Norberto Sánchez-Cruz, José L. Medina-Franco, and Ronald J. Quinn. 2020. "Lessons from Exploring Chemical Space and Chemical Diversity of Propolis Components" International Journal of Molecular Sciences 21, no. 14: 4988. https://doi.org/10.3390/ijms21144988
APA StyleTran, T. D., Ogbourne, S. M., Brooks, P. R., Sánchez-Cruz, N., Medina-Franco, J. L., & Quinn, R. J. (2020). Lessons from Exploring Chemical Space and Chemical Diversity of Propolis Components. International Journal of Molecular Sciences, 21(14), 4988. https://doi.org/10.3390/ijms21144988