Selective Gold Recovery from Homogenous Aqueous Solutions Containing Gold and Platinum Ions by Aromatic Amino Acid-Containing Peptides
Abstract
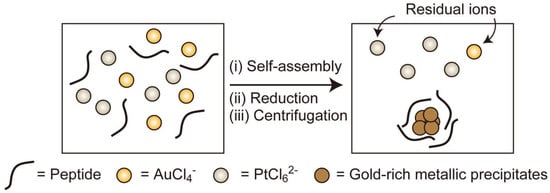
:1. Introduction
2. Experimental Section
2.1. General
2.2. Preparation of Known Peptides
2.3. Peptide Synthesis and Storage
2.4. Attenuated Total Reflectance Fourier-Transform Infrared Spectroscopy (ATR-FTIR)
2.5. Reduction of Noble Metal Ions with Peptides
2.6. Measurement of Absorption Spectra of Reaction Mixtures
2.7. Field Emission-Scanning Electron Microscopy (FE-SEM) and Energy-Dispersive X-ray Spectroscopy-Scanning Electron Microscopy (EDS-SEM)
2.8. Transmission Electron Microscopy (TEM)
2.9. Inductively Coupled Plasma-Optical Emission Spectroscopy (ICP-OES)
2.10. Thermal Gravimetric Analysis (TGA) of the Precipitates
3. Results and Discussion
3.1. Design, Synthesis and Characterization of the Peptides
3.2. Reduction of Metal Ions by the Peptides
3.3. Characterization of Precipitates from Peptide/Noble Metal Reaction Mixtures
3.4. Selectivity of the Peptide-Based Recovery System for Gold
3.5. Mechanism of Selective Gold Recovery by the Peptide-Based System
3.6. Role of Peptide Self-Assembly for Selective Gold Recovery
3.7. Gold Recovery Using Common Reducing Agents
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kaya, M. Recovery of Materials and Nonmaterials from Electronic Wastes by Physical and Chemical Recycling Processes. Waste Manag. 2016, 57, 64–90. [Google Scholar] [CrossRef] [PubMed]
- Cox, M. Solvent Extraction in Hydrometallurgy. In Solvent Extraction Principles and Practice, 2nd ed.; Revised and Expanded; Rydberg, J., Cox, M., Musikas, C., Choppin, G.R., Eds.; Marcel Dekker, Inc.: New York, NY, USA; Basel, Switzerland, 2002; pp. 455–505. [Google Scholar]
- Won, S.W.; Kotte, P.; Wei, W.; Lim, A.; Yum, Y.S. Biosorbents for Recovery of Precious Metals. Biosens. Technol. 2014, 160, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Syed, S. Recovery of Gold from Secondary Sources-A Review. Hydrometallurgy 2012, 115–116, 30–51. [Google Scholar] [CrossRef]
- Kim, J.; Kim, K.R.; Hong, Y.; Choi, S.; Yavuz, C.T.; Kim, J.W.; Nam, Y.S. Photochemically Enhanced Selective Adsorption of Gold Ions on Tannin-Coated Poros Polymer Microspheres. ACS Appl. Mater. Interfaces 2019, 11, 21915–21925. [Google Scholar] [CrossRef]
- Daniel, M.-C.; Astruc, D. Gold Nanoparticles: Assembly, Supramolecular Chemistry, Quantum-Size-Related Properties, and Applications toward Biology, Catalysis, and Nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef]
- Eustis, S.; El-Sayed, M.A. Why Gold Nanoparticles Are More Precious than Pretty Gold: Noble Metal Surface Plasmon Resonance and Its Enhancement of the Radiative and Nonradiative Properties of Nanocrystals of Different Shapes. Chem. Soc. Rev. 2006, 35, 209–217. [Google Scholar] [CrossRef]
- Hu, M.; Chen, J.; Li, Z.-Y.; Au, L.; Hartland, G.V.; Li, X.; Marquez, M.; Xia, Y. Gold Nanostructures: Engineering Their Plasmonic Properties for Biomedical Applications. Chem. Soc. Rev. 2006, 35, 1084–1094. [Google Scholar] [CrossRef]
- Cobley, C.M.; Chen, J.; Cho, E.C.; Wang, L.V.; Xia, Y. Gold Nanostructures: A Class of Multifunctional Materials for Biomedical Applications. Chem. Soc. Rev. 2011, 40, 44–56. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, S.K.; Pal, T. Interparticle Coupling Effect on the Surface Plasmon Resonance of Gold Nanoparticles: From Theory to Applications. Chem. Rev. 2007, 107, 4797–4862. [Google Scholar] [CrossRef]
- Jain, P.K.; Huang, X.; El-Sayed, I.H.; El-Sayed, M.A. Noble Metals on the Nanoscale: Optical and Photothermal Properties and Some Applications in Imaging, Sensing, Biology, and Medicine. Acc. Chem. Res. 2008, 41, 1578–1586. [Google Scholar] [CrossRef]
- Murphy, C.J.; Gole, A.M.; Stone, J.W.; Sisco, P.N.; Alkilany, A.M.; Goldsmith, E.C.; Baxter, S.C. Gold Nanoparticles in Biology: Beyond Toxicity to Cellular Imaging. Acc. Chem. Res. 2008, 41, 1721–1730. [Google Scholar] [CrossRef] [PubMed]
- Sperling, R.A.; Gil, P.R.; Zhang, F.; Zanella, M.; Parak, W.J. Biological Applications of Gold Nanoparticles. Chem. Soc. Rev. 2008, 37, 1896–1908. [Google Scholar] [CrossRef]
- Boisselier, E.; Astruc, D. Gold Nanoparticles in Nanomedicine: Preparations, Imaging, Diagnostics, Therapies and Toxicity. Chem. Soc. Rev. 2009, 38, 1759–1782. [Google Scholar] [CrossRef] [PubMed]
- Sau, T.K.; Rogach, A.L.; Jackel, F.; Klar, T.A.; Feldmann, J. Properties and Applications of Colloidal Nonspherical Noble Metal Nanoparticles. Adv. Mater. 2010, 22, 1805–1825. [Google Scholar] [CrossRef]
- Slepička, P.; Kasálková, N.S.; Siegel, J.; Kolská, Z.; Švorčík, V. Methods of Gold and Silver Nanoparticle Preparation. Materials 2020, 13, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuo, C.-H.; Huang, M. Synthesis of Branched Gold Nanocrystals by a Seeding Growth Approach. Langmuir 2005, 21, 2012–2016. [Google Scholar] [CrossRef]
- Tomizaki, K.-Y.; Wakizaka, S.; Yamaguchi, Y.; Kobayashi, A.; Imai, T. Ultrathin Gold Nanoribbons Synthesized within the Interior Cavity of a Self-Assembled Peptide Nanoarchitecture. Langmuir 2014, 30, 846–856. [Google Scholar] [CrossRef]
- Tomizaki, K.-Y.; Kishioka, K.; Kobayashi, H.; Kobayashi, A.; Yamada, N.; Kataoka, S.; Imai, T.; Kasuno, M. Roles of Aromatic Side Chains and Template Effects of the Hydrophobic Cavity of a Self-Assembled Peptide Nanoarchitecture for Anisotropic Growth of Gold Nanocrystals. Bioorg. Med. Chem. 2015, 23, 7282–7291. [Google Scholar] [CrossRef]
- Tomizaki, K.-Y.; Kishioka, K.; Kataoka, S.; Miyatani, M.; Ikeda, T.; Komada, M.; Imai, T.; Usui, K. Non-Covalent Loading of Anti-Cancer Doxorubicin by Modularizable Peptide Self-Assemblies for a Nanoscale Drug Carrier. Molecules 2017, 22, 1916. [Google Scholar] [CrossRef] [Green Version]
- Kasuno, K.; Morishima, K.; Matsushita, T.; Kihara, S. Development of a Simple Column Electrode for Sensitive and Rapid Coulometry. Anal. Sci. 2009, 25, 941–945. [Google Scholar] [CrossRef] [Green Version]
- Chan, W.C.; White, P.D. Fmoc Solid Phase Peptide Synthesis: A Practical Approach; Oxford University Press: New York, NY, USA, 2000; pp. 41–76. [Google Scholar]
- Dong, A.; Huang, P.; Caughey, W.S. Protein Secondary Structures in Water from Second-Derivative Amide I Infrared Spectra. Biochemistry 1990, 29, 3303–3308. [Google Scholar] [CrossRef] [PubMed]
- Srisailam, S.; Wang, H.-M.; Kumar, T.K.S.; Rajalingam, D.; Sivaraja, V.; Sheu, H.-S.; Chang, Y.-C.; Yu, C. Amyloid-Like Fibril Formation in an All Beta-Barrel Protein Involves the Formation of Partially Structured Intermediate(s). J. Biol. Chem. 2002, 277, 19027–19036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sethi, M.; Knecht, M.R. Understanding the Mechanism of Amino Acid-Based Au Nanoparticle Chain Formation. Langmuir 2010, 26, 9860–9874. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.A.; Hughes, Z.E.; Lin, Y.; Li, Y.; Swihart, M.T.; Knecht, M.R.; Walsh, T.R. Peptide-Mediated Growth and Dispersion of Au Nanoparticles in Water via Sequence Engineering. J. Phys. Chem. C 2018, 122, 11532–11542. [Google Scholar] [CrossRef] [Green Version]
- Yan, J.; Pan, Y.; Cheetham, A.G.; Lin, Y.-A.; Wang, W.; Cui, H.; Liu, C.-J. One-Step Fabrication of Self-Assembled Peptide Thin Films with Highly Dispersed Noble Metal Nanoparticles. Langmuir 2013, 29, 16051–16057. [Google Scholar] [CrossRef]
- Ataee-Esfahani, H.; Wang, L.; Nemoto, Y.; Yamauchi, Y. Synthesis of Bimetallic Au@Pt Nanoparticles with Au core and Nanostructured Pt Shell toward Highly Active Electrocatalysts. Chem. Mater. 2010, 22, 6310–6318. [Google Scholar] [CrossRef]
- Guo, S.; Li, J.; Dong, S.; Wang, E. Three-Dimensional Pt-on-Au Bimetallic Dendritic nanoparticle: One-Step, High-Yield Synthesis and Its Bifunctional Plasmonic and Catalytic Properties. J. Phys. Chem. C 2010, 114, 15337–15342. [Google Scholar] [CrossRef]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomizaki, K.-y.; Okamoto, T.; Tonoda, T.; Imai, T.; Asano, M. Selective Gold Recovery from Homogenous Aqueous Solutions Containing Gold and Platinum Ions by Aromatic Amino Acid-Containing Peptides. Int. J. Mol. Sci. 2020, 21, 5060. https://doi.org/10.3390/ijms21145060
Tomizaki K-y, Okamoto T, Tonoda T, Imai T, Asano M. Selective Gold Recovery from Homogenous Aqueous Solutions Containing Gold and Platinum Ions by Aromatic Amino Acid-Containing Peptides. International Journal of Molecular Sciences. 2020; 21(14):5060. https://doi.org/10.3390/ijms21145060
Chicago/Turabian StyleTomizaki, Kin-ya, Takuya Okamoto, Tatsuki Tonoda, Takahito Imai, and Masahiro Asano. 2020. "Selective Gold Recovery from Homogenous Aqueous Solutions Containing Gold and Platinum Ions by Aromatic Amino Acid-Containing Peptides" International Journal of Molecular Sciences 21, no. 14: 5060. https://doi.org/10.3390/ijms21145060
APA StyleTomizaki, K. -y., Okamoto, T., Tonoda, T., Imai, T., & Asano, M. (2020). Selective Gold Recovery from Homogenous Aqueous Solutions Containing Gold and Platinum Ions by Aromatic Amino Acid-Containing Peptides. International Journal of Molecular Sciences, 21(14), 5060. https://doi.org/10.3390/ijms21145060