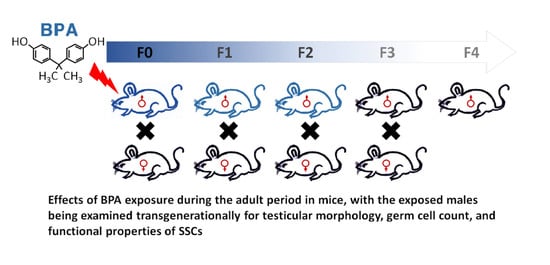
Paternal Exposure to Bisphenol-A Transgenerationally Impairs Testis Morphology, Germ Cell Associations, and Stemness Properties of Mouse Spermatogonial Stem Cells
Abstract
:1. Introduction
2. Results
2.1. BPA Increases the Frequency of Abnormal Seminiferous Tubules (STs) in F0–F2 Generations
2.2. BPA Changes the Size of SE and Alters Stages of STs
2.3. BPA-Induced Alterations in the Count of Spermatogonia
2.4. BPA Exposure Induces Germ Cell Apoptosis
2.5. BPA Affects the Stemness Properties of SSCs in F2 Offspring
3. Discussion
4. Materials and Methods
4.1. Experimental Animals
4.2. Experimental Design, BPA Exposure, and Breeding to Generate F1–F4 Offspring
4.3. Collection of Testes and Determination of Testicular Abnormalities
4.4. Periodic Acid–Schiff (PAS)–H&E Staining, Staging of SE, and Counting of Spermatogonia
4.5. Flow Cytometric Analysis
4.6. Detection of Germ Cell Apoptosis
4.7. Germ Cell Transplantation to Evaluate the Activity of SSCs
4.8. Statistics
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lang, I.A.; Galloway, T.S.; Scarlett, A.; Henley, W.E.; Depledge, M.; Wallace, R.B.; Melzer, D. Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults. JAMA 2008, 300, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Vandenberg, L.N.; Chahoud, I.; Heindel, J.J.; Padmanabhan, V.; Paumgartten, F.J.; Schoenfelder, G. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environ. Health Perspect. 2010, 118, 1055–1070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le, H.H.; Carlson, E.M.; Chua, J.P.; Belcher, S.M. Bisphenol A is released from polycarbonate drinking bottles and mimics the neurotoxic actions of estrogen in developing cerebellar neurons. Toxicol. Lett. 2008, 176, 149–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maragou, N.C.; Makri, A.; Lampi, E.N.; Thomaidis, N.S.; Koupparis, M.A. Migration of bisphenol A from polycarbonate baby bottles under real use conditions. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2008, 25, 373–383. [Google Scholar] [CrossRef] [Green Version]
- Richter, C.A.; Birnbaum, L.S.; Farabollini, F.; Newbold, R.R.; Rubin, B.S.; Talsness, C.E.; Vandenbergh, J.G.; Walser-Kuntz, D.R.; vom Saal, F.S. V In vivo effects of bisphenol A in laboratory rodent studies. Reprod. Toxicol. 2007, 24, 199–224. [Google Scholar] [CrossRef] [Green Version]
- Vandenberg, L.N.; Hauser, R.; Marcus, M.; Olea, N.; Welshons, W. V Human exposure to bisphenol A (BPA). Reprod. Toxicol. 2007, 24, 139–177. [Google Scholar] [CrossRef]
- Souter, I.; Smith, K.W.; Dimitriadis, I.; Ehrlich, S.; Williams, P.L.; Calafat, A.M.; Hauser, R. The association of bisphenol-A urinary concentrations with antral follicle counts and other measures of ovarian reserve in women undergoing infertility treatments. Reprod. Toxicol. 2013, 42, 224–231. [Google Scholar] [CrossRef] [Green Version]
- Meeker, J.D.; Ehrlich, S.; Toth, T.L.; Wright, D.L.; Calafat, A.M.; Trisini, A.T.; Ye, X.; Hauser, R. Semen quality and sperm DNA damage in relation to urinary bisphenol A among men from an infertility clinic. Reprod. Toxicol. 2010, 30, 532–539. [Google Scholar] [CrossRef] [Green Version]
- Cummings, A.M.; Laws, S.C. Assessment of estrogenicity by using the delayed implanting rat model and examples. Reprod. Toxicol. 2000, 14, 111–117. [Google Scholar] [CrossRef]
- Krishnan, A.V.; Stathis, P.; Permuth, S.F.; Tokes, L.; Feldman, D. Bisphenol-A: An Estrogenic Substance Is Released from Polycarbonate Flasks during Autoclaving. Endocrinology 1993, 132, 2279–2286. [Google Scholar] [CrossRef]
- Rubin, B.S. Bisphenol A: An endocrine disruptor with widespread exposure and multiple effects. J. Steroid Biochem. Mol. Biol. 2011, 127, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Morrisey, R.E.; George, J.D.; Price, C.J.; Tyl, R.W.; Marr, M.C.; Kimmel, C.A. The Developmental Toxicity of Bisphenol-a in Rats and Mice. Fundam. Appl. Toxicol. 1987, 8, 571–582. [Google Scholar] [CrossRef]
- Bindhumol, V.; Chitra, K.C.; Mathur, P.P. Bisphenol A induces reactive oxygen species generation in the liver of male rats. Toxicology 2003, 188, 117–124. [Google Scholar] [CrossRef]
- Mustieles, V.; Perez-Lobato, R.; Olea, N.; Fernandez, M.F. Bisphenol A: Human exposure and neurobehavior. Neurotoxicology 2015, 49, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Ranciere, F.; Lyons, J.G.; Loh, V.H.Y.; Botton, J.; Galloway, T.; Wang, T.G.; Shaw, J.E.; Magliano, D.J. Bisphenol A and the risk of cardiometabolic disorders: A systematic review with meta-analysis of the epidemiological evidence. Environ. Health 2015, 14, 46. [Google Scholar] [CrossRef] [Green Version]
- Urriola-Munoz, P.; Lagos-Cabre, R.; Moreno, R.D. A Mechanism of Male Germ Cell Apoptosis Induced by Bisphenol-A and Nonylphenol Involving ADAM17 and p38 MAPK Activation. PLoS ONE 2014, 9, e113793. [Google Scholar] [CrossRef] [Green Version]
- Brinster, R.L. Male germline stem cells: From mice to men. Science 2007, 316, 404–405. [Google Scholar] [CrossRef] [Green Version]
- Oatley, J.M.; Brinster, R.L. Regulation of Spermatogonial Stem Cell Self-Renewal in Mammals. Annu. Rev. Cell Dev. Biol. 2008, 24, 263–286. [Google Scholar] [CrossRef] [Green Version]
- Vandenberg, L.N.; Maffini, M.V.; Sonnenschein, C.; Rubin, B.S.; Soto, A.M. Bisphenol-A and the Great Divide: A Review of Controversies in the Field of Endocrine Disruption. Endocr. Rev. 2009, 30, 75–95. [Google Scholar] [CrossRef]
- Li, J.; Mao, R.; Zhou, Q.; Ding, L.; Tao, J.; Ran, M.M.; Gao, E.S.; Yuan, W.; Wang, J.T.; Hou, L.F. Exposure to bisphenol A (BPA) in Wistar rats reduces sperm quality with disruption of ERK signal pathway. Toxicol. Mech. Methods 2016, 26, 180–188. [Google Scholar] [CrossRef]
- Tiwari, D.; Vanage, G. Mutagenic effect of Bisphenol A on adult rat male germ cells and their fertility. Reprod. Toxicol. 2013, 40, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S.; Kwon, W.S.; Lee, J.S.; Yoon, S.J.; Ryu, B.Y.; Pang, M.G. Bisphenol-A Affects Male Fertility via Fertility-related Proteins in Spermatozoa. Sci. Rep. 2015, 5, 9196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, M.S.; Kwon, W.S.; Yoon, S.J.; Park, Y.J.; Ryu, B.Y.; Pang, M.G. A novel approach to assessing bisphenol-A hazards using an in vitro model system. BMC Genom. 2016, 17, 577. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Duan, W.; Li, R.; Xu, S.; Zhang, L.; Chen, C.; He, M.; Lu, Y.; Wu, H.; Pi, H.; et al. Exposure to bisphenol A disrupts meiotic progression during spermatogenesis in adult rats through estrogen-like activity. Cell Death Dis. 2013, 4, e676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vrooman, L.A.; Oatley, J.M.; Griswold, J.E.; Hassold, T.J.; Hunt, P.A. Estrogenic Exposure Alters the Spermatogonial Stem Cells in the Developing Testis, Permanently Reducing Crossover Levels in the Adult. PLoS Genet. 2015, 11, e1004949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doshi, T.; D’souza, C.; Vanage, G. Aberrant DNA methylation at Igf2-H19 imprinting control region in spermatozoa upon neonatal exposure to bisphenol A and its association with post implantation loss. Mol. Biol. Rep. 2013, 40, 4747–4757. [Google Scholar] [CrossRef]
- Hulak, M.; Gazo, I.; Shaliutina, A.; Linhartova, P. In vitro effects of bisphenol A on the quality parameters, oxidative stress, DNA integrity and adenosine triphosphate content in sterlet (Acipenser ruthenus) spermatozoa. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2013, 158, 64–71. [Google Scholar] [CrossRef]
- Bolt, H.M.; Stewart, J.D. Highlight report: The bisphenol A controversy. Arch. Toxicol. 2011, 85, 1491–1492. [Google Scholar] [CrossRef] [Green Version]
- Karmakar, P.C.; Kang, H.G.; Kim, Y.H.; Jung, S.E.; Rahman, M.S.; Lee, H.S.; Kim, Y.H.; Pang, M.G.; Ryu, B.Y. Bisphenol A Affects on the Functional Properties and Proteome of Testicular Germ Cells and Spermatogonial Stem Cells in vitro Culture Model. Sci. Rep. 2017, 7, 11858. [Google Scholar] [CrossRef]
- Ge, L.C.; Chen, Z.J.; Liu, H.; Zhang, K.S.; Su, Q.; Ma, X.Y.; Huang, H.B.; Zhao, Z.D.; Wang, Y.Y.; Giesy, J.P.; et al. Signaling related with biphasic effects of bisphenol A (BPA) on Sertoli cell proliferation: A comparative proteomic analysis. Biochim. Biophys. Acta General Subj. 2014, 1840, 2663–2673. [Google Scholar] [CrossRef]
- Rahman, M.S.; Kwon, W.S.; Karmakar, P.C.; Yoon, S.J.; Ryu, B.Y.; Pang, M.G. Gestational Exposure to Bisphenol A Affects the Function and Proteome Profile of F1 Spermatozoa in Adult Mice. Environ. Health Perspect. 2017, 125, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.N.; Bu, P.L.; Li, F.J.; Lan, S.J.; Wu, H.J.; Yuan, L.; Wang, Y. Neonatal bisphenol A exposure induces meiotic arrest and apoptosis of spermatogenic cells. Oncotarget 2016, 7, 10606–10615. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Luo, C.; Li, Q.; Chen, S.; Hu, Y. Mitochondrion-mediated apoptosis is involved in reproductive damage caused by BPA in male rats. Environ. Toxicol. Pharmacol. 2014, 38, 1025–1033. [Google Scholar] [CrossRef] [PubMed]
- Anjum, S.; Rahman, S.; Kaur, M.; Ahmad, F.; Rashid, H.; Ansari, R.A.; Raisuddin, S. Melatonin ameliorates bisphenol A-induced biochemical toxicity in testicular mitochondria of mouse. Food Chem. Toxicol. 2011, 49, 2849–2854. [Google Scholar] [CrossRef] [PubMed]
- Sakaue, M.; Ohsako, S.; Ishimura, R.; Kurosawa, S.; Kurohmaru, M.; Hayashi, Y.; Aoki, Y.; Yonemoto, J.; Tohyama, C. Bisphenol-A affects spermatogenesis in the adult rat even at a low dose. J. Occup. Health 2001, 43, 185–190. [Google Scholar] [CrossRef] [Green Version]
- Vom Saal, F.S.; Cooke, P.S.; Buchanan, D.L.; Palanza, P.; Thayer, K.A.; Nagel, S.C.; Parmigiani, S.; Welshons, W.V. A Physiologically Based Approach To the Study of Bisphenol a and Other Estrogenic Chemicals On the Size of Reproductive Organs, Daily Sperm Production, and Behavior. Toxicol. Ind. Health 1998, 14, 239–260. [Google Scholar] [CrossRef] [PubMed]
- Wing, T.Y.; Christensen, A.K. Morphometric Studies on Rat Seminiferous Tubules. Am. J. Anat. 1982, 165, 13–25. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, E.A.; de Rooij, D.G. Staging of mouse seminiferous tubule cross-sections. Methods Mol. Biol. 2009, 558, 263–277. [Google Scholar] [CrossRef]
- Timms, B.G.; Howdeshell, K.L.; Barton, L.; Bradley, S.; Richter, C.A.; vom Saal, F.S. Estrogenic chemicals in plastic and oral contraceptives disrupt development of the fetal mouse prostate and urethra. Proc. Natl. Acad. Sci. USA 2005, 102, 7014–7019. [Google Scholar] [CrossRef] [Green Version]
- Doyle, T.J.; Bowman, J.L.; Windell, V.L.; McLean, D.J.; Kim, K.H. Transgenerational Effects of Di-(2-ethylhexyl) Phthalate on Testicular Germ Cell Associations and Spermatogonial Stem Cells in Mice. Biol. Reprod. 2013, 88, 1–15. [Google Scholar] [CrossRef]
- Oatley, J.M.; Brinster, R.L. Spermatogonial stem cells. Adult Stem Cells 2006, 419, 259–282. [Google Scholar] [CrossRef]
- Brinster, R.L.; Zimmermann, J.W. Spermatogenesis Following Male Germ-Cell Transplantation. Proc. Natl. Acad. Sci. USA 1994, 91, 11298–11302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tagelenbosch, R.A.J.; de Rooij, D.G. A quantitative study of spermatogonial multiplication and stem cell renewal in the C3H/101 F1 hybrid mouse. Mutat. Res. Fundam. Mol. Mech. Mutagen. 1993, 290, 193–200. [Google Scholar] [CrossRef]
- Nagano, M.; Avarbock, M.R.; Brinster, R.L. Pattern and Kinetics of Mouse Donor Spermatogonial Stem Cell Colonization in Recipient Testes. Biol. Reprod. 1999, 60, 1429–1436. [Google Scholar] [CrossRef] [Green Version]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karmakar, P.C.; Ahn, J.S.; Kim, Y.-H.; Jung, S.-E.; Kim, B.-J.; Lee, H.-S.; Kim, S.-U.; Rahman, M.S.; Pang, M.-G.; Ryu, B.-Y. Paternal Exposure to Bisphenol-A Transgenerationally Impairs Testis Morphology, Germ Cell Associations, and Stemness Properties of Mouse Spermatogonial Stem Cells. Int. J. Mol. Sci. 2020, 21, 5408. https://doi.org/10.3390/ijms21155408
Karmakar PC, Ahn JS, Kim Y-H, Jung S-E, Kim B-J, Lee H-S, Kim S-U, Rahman MS, Pang M-G, Ryu B-Y. Paternal Exposure to Bisphenol-A Transgenerationally Impairs Testis Morphology, Germ Cell Associations, and Stemness Properties of Mouse Spermatogonial Stem Cells. International Journal of Molecular Sciences. 2020; 21(15):5408. https://doi.org/10.3390/ijms21155408
Chicago/Turabian StyleKarmakar, Polash Chandra, Jin Seop Ahn, Yong-Hee Kim, Sang-Eun Jung, Bang-Jin Kim, Hee-Seok Lee, Sun-Uk Kim, Md Saidur Rahman, Myung-Geol Pang, and Buom-Yong Ryu. 2020. "Paternal Exposure to Bisphenol-A Transgenerationally Impairs Testis Morphology, Germ Cell Associations, and Stemness Properties of Mouse Spermatogonial Stem Cells" International Journal of Molecular Sciences 21, no. 15: 5408. https://doi.org/10.3390/ijms21155408
APA StyleKarmakar, P. C., Ahn, J. S., Kim, Y. -H., Jung, S. -E., Kim, B. -J., Lee, H. -S., Kim, S. -U., Rahman, M. S., Pang, M. -G., & Ryu, B. -Y. (2020). Paternal Exposure to Bisphenol-A Transgenerationally Impairs Testis Morphology, Germ Cell Associations, and Stemness Properties of Mouse Spermatogonial Stem Cells. International Journal of Molecular Sciences, 21(15), 5408. https://doi.org/10.3390/ijms21155408