The Effects of Cannabidiol and Prognostic Role of TRPV2 in Human Endometrial Cancer
Abstract
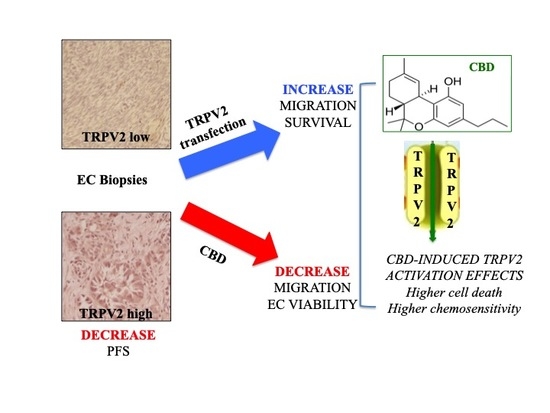
:1. Introduction
2. Results
2.1. CB Receptors and TRPVs Gene Expression in EC Samples from TCGA
2.2. TRPV2 Expression Increased with the Increasing of Non-Endometrioid Component
2.3. TRPV2 Expression Increased with the Malignancy of Type II EC and Correlated with a Shorter PFS
2.4. TRPV2 Expression Stimulated Migration and Survival of EC Cells
2.5. TRPV2 Expression Influenced the Effect of Chemotherapeutic Drugs
2.6. CBD Induced Cytotoxicity in EC Cell Lines in Single and Daily Administration
2.7. CBD Induced Cell Death in Type I EC Cell Lines
2.8. CBD Induced Cell Cycle Arrest and Autophagy in Mixed Type I/II EC Cell Lines
2.9. CBD Inhibited Migratory Ability of EC Cells
2.10. CBD Increased the Effect of Chemotherapeutic Drugs Used for EC Treatment
3. Discussion
4. Materials and Methods
4.1. The Cancer Genome Atlas (TCGA) and cBioportal Database Analysis
4.2. Endometrial Cancer Cell Lines
4.3. Materials
4.4. RNA Isolation, Reverse Transcription and Quantitative Real-Time PCR
4.5. Western Blot Analysis
4.6. Patient Samples
4.7. Immunohistochemical Stainings
4.8. Evaluation and Scoring of Immunohistochemical Stainings
4.9. Cell Transfection
4.10. Wound-Healing Assay
4.11. 3-[4,5-Dimethylthiazol-2-Yl]-2,5 Diphenyl Tetrazolium Bromide (MTT) Assay
4.12. Apoptosis Assays and PI Staining
4.13. Cell Cycle Analysis
4.14. Acridine Orange Staining
4.15. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Davis, M.P. Cannabinoids for Symptom Management and Cancer Therapy: The Evidence. J. Natl. Compr. Cancer Netw. 2016, 14, 915–922. [Google Scholar] [CrossRef] [PubMed]
- Kleckner, A.S.; Kleckner, I.R.; Kamen, C.S.; Tejani, M.A.; Janelsins, M.C.; Morrow, G.R.; Peppone, L.J. Opportunities for cannabis in supportive care in cancer. Ther. Adv. Med. Oncol. 2019, 11, 1758835919866362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hinz, B.; Ramer, R. Anti-tumour actions of cannabinoids. Br. J. Pharmacol. 2019, 176, 1384–1394. [Google Scholar] [CrossRef] [PubMed]
- Nabissi, M.; Morelli, M.B.; Amantini, C.; Liberati, S.; Santoni, M.; Ricci-Vitiani, L.; Pallini, R.; Santoni, G. Cannabidiol stimulates Aml-1a-dependent glial differentiation and inhibits glioma stem-like cells proliferation by inducing autophagy in a TRPV2-dependent manner. Int. J. Cancer 2015, 137, 8–69. [Google Scholar] [CrossRef] [Green Version]
- Ivanov, V.N.; Wu, J.; Hei, T.K. Regulation of human glioblastoma cell death by combined treatment of cannabidiol, γ-radiation and small molecule inhibitors of cell signalling pathways. Oncotarget 2017, 8, 74068–74095. [Google Scholar] [CrossRef] [Green Version]
- Vaccani, A.; Massi, P.; Colombo, A.; Rubino, T.; Parolaro, D. Cannabidiol inhibits human glioma cell migration through a cannabinoid receptor-independent mechanism. Br. J. Pharmacol. 2005, 144, 1032–1036. [Google Scholar] [CrossRef] [Green Version]
- Ramer, R.; Heinemann, K.; Merkord, J.; Rohde, H.; Salamon, A.; Linnebacher, M.; Hinz, B. COX-2 and PPAR-γ confer cannabidiol-induced apoptosis of human lung cancer cells. Mol. Cancer Ther. 2013, 12, 69–82. [Google Scholar] [CrossRef] [Green Version]
- Ramer, R.; Bublitz, K.; Freimuth, N.; Merkord, J.; Rohde, H.; Haustein, M.; Borchert, P.; Schmuhl, E.; Linnebacher, M.; Hinz, B. Cannabidiol inhibits lung cancer cell invasion and metastasis via intercellular adhesion molecule-1. FASEB J. 2012, 26, 1535–1548. [Google Scholar] [CrossRef] [Green Version]
- Ramer, R.; Rohde, A.; Merkord, J.; Rohde, H.; Hinz, B. Decrease of plasminogen activator inhibitor-1 may contribute to the anti-invasive action of cannabidiol on human lung cancer cells. Pharm. Res. 2010, 27, 2162–2174. [Google Scholar] [CrossRef]
- Zhang, X.; Qin, Y.; Pan, Z.; Li, M.; Liu, X.; Chen, X.; Qu, G.; Zhou, L.; Xu, M.; Zheng, Q.; et al. Cannabidiol Induces Cell Cycle Arrest and Cell Apoptosis in Human Gastric Cancer SGC-7901 Cells. Biomolecules 2019, 9, 302. [Google Scholar] [CrossRef] [Green Version]
- Sultan, A.S.; Marie, M.A.; Sheweita, S.A. Novel mechanism of cannabidiol-induced apoptosis in breast cancer cell lines. Breast 2018, 41, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, A.; Kuzontkoski, P.M.; Groopman, J.E.; Prasad, A. Cannabidiol induces programmed cell death in breast cancer cells by coordinating the cross-talk between apoptosis and autophagy. Mol. Cancer Ther. 2011, 10, 7–1172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elbaz, M.; Nasser, M.W.; Ravi, J.; Wani, N.A.; Ahirwar, D.K.; Zhao, H.; Oghumu, S.; Satoskar, A.R.; Shilo, K.; Carson, W.E., 3rd; et al. Modulation of the tumor microenvironment and inhibition of EGF/EGFR pathway: Novel anti-tumor mechanisms of Cannabidiol in breast cancer. Mol. Oncol. 2015, 9, 906–919. [Google Scholar] [CrossRef] [Green Version]
- Simmerman, E.; Qin, X.; Yu, J.C.; Baban, B. Cannabinoids as a Potential New and Novel Treatment for Melanoma: A Pilot Study in a Murine Model. J. Surg. Res. 2019, 235, 210–215. [Google Scholar] [CrossRef] [PubMed]
- De Petrocellis, L.; Ligresti, A.; Schiano Moriello, A.; Iappelli, M.; Verde, R.; Stott, C.G.; Cristino, L.; Orlando, P.; Di Marzo, V. Non-THC cannabinoids inhibit prostate carcinoma growth in vitro and in vivo: Pro-apoptotic effects and underlying mechanisms. Br. J. Pharmacol. 2013, 168, 79–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aviello, G.; Romano, B.; Borrelli, F.; Capasso, R.; Gallo, L.; Piscitelli, F.; Di Marzo, V.; Izzo, A.A. Chemopreventive effect of the non-psychotropic phytocannabinoid cannabidiol on experimental colon cancer. J. Mol. Med. 2012, 90, 925–934. [Google Scholar] [CrossRef]
- Sreevalsan, S.; Joseph, S.; Jutooru, I.; Chadalapaka, G.; Safe, S.H. Induction of apoptosis by cannabinoids in prostate and colon cancer cells is phosphatase dependent. Anticancer Res. 2011, 31, 3799–3807. [Google Scholar]
- Fisher, T.; Golan, H.; Schiby, G.; PriChen, S.; Smoum, R.; Moshe, I.; Peshes-Yaloz, N.; Castiel, A.; Waldman, D.; Gallily, R.; et al. In vitro and in vivo efficacy of non-psychoactive cannabidiol in neuroblastoma. Curr. Oncol. 2016, 23, S15–S22. [Google Scholar] [CrossRef] [Green Version]
- Morelli, M.B.; Offidani, M.; Alesiani, F.; Discepoli, G.; Liberati, S.; Olivieri, A.; Santoni, M.; Santoni, G.; Leoni, P.; Nabissi, M. The effects of cannabidiol and its synergism with bortezomib in multiple myeloma cell lines. A role for transient receptor potential vanilloid type-2. Int. J. Cancer 2014, 134, 2534–2546. [Google Scholar] [CrossRef]
- Nabissi, M.; Morelli, M.B.; Offidani, M.; Amantini, C.; Gentili, S.; Soriani, A.; Cardinali, C.; Leoni, P.; Santoni, G. Cannabinoids synergize with carfilzomib, reducing multiple myeloma cells viability and migration. Oncotarget 2016, 7, 77547–77557. [Google Scholar] [CrossRef] [Green Version]
- Gallily, R.; Even-Chena, T.; Katzavian, G.; Lehmann, D.; Dagan, A.; Mechoulam, R. Gamma-irradiation enhances apoptosis induced by cannabidiol, a non-psychotropic cannabinoid, in cultured HL-60 myeloblastic leukemia cells. Leuk. Lymphoma 2003, 44, 10–1773. [Google Scholar] [CrossRef] [PubMed]
- McKallip, R.J.; Jia, W.; Schlomer, J.; Warren, J.W.; Nagarkatti, P.S.; Nagarkatti, M. Cannabidiol-induced apoptosis in human leukemia cells: A novel role of cannabidiol in the regulation of p22phox and Nox4 expression. Mol. Pharmacol. 2006, 70, 897–908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharafi, G.; He, H.; Nikfarjam, M. Potential Use of Cannabinoids for the Treatment of Pancreatic Cancer. J. Pancreat. Cancer. 2019, 5, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, B.M.; Correia-da-Silva, G.; Teixeira, N.A. Cannabinoid-induced cell death in endometrial cancer cells: Involvement of TRPV1 receptors in apoptosis. J. Physiol. Biochem. 2018, 74, 2–272. [Google Scholar] [CrossRef] [PubMed]
- Nabissi, M.; Morelli, M.B.; Santoni, M.; Santoni, G. Triggering of the TRPV2 channel by cannabidiol sensitizes glioblastoma cells to cytotoxic chemotherapeutic agents. Carcinogenesis 2013, 34, 48–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morales, P.; Hurst, D.P.; Reggio, P.H. Molecular Targets of the Phytocannabinoids: A Complex Picture. Prog. Chem. Org. Nat. Prod. 2017, 103, 103–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Petrocellis, L.; Ligresti, A.; Moriello, A.S.; Allarà, M.; Bisogno, T.; Petrosino, S.; Stott, C.G.; Di Marzo, V. Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br. J. Pharmacol. 2011, 163, 1479–1494. [Google Scholar] [CrossRef] [Green Version]
- Muller, C.; Morales, P.; Reggio, P.H. Cannabinoid Ligands Targeting TRP Channels. Front. Mol. Neurosci. 2019, 11, 487. [Google Scholar] [CrossRef]
- Pellati, F.; Borgonetti, V.; Brighenti, V.; Biagi, M.; Benvenuti, S.; Corsi, L. Cannabis sativa L. and Nonpsychoactive Cannabinoids: Their Chemistry and Role against Oxidative Stress, Inflammation, and Cancer. Biomed. Res. Int. 2018, 2018, 1691428. [Google Scholar] [CrossRef] [Green Version]
- Santoni, G.; Amantini, C.; Maggi, F.; Marinelli, O.; Santoni, M.; Nabissi, M.; Morelli, M.B. The TRPV2 cation channels: From urothelial cancer invasiveness to glioblastoma multiforme interactome signature. Lab. Investig. 2020, 100, 186–198. [Google Scholar] [CrossRef]
- Caprodossi, S.; Lucciarini, R.; Amantini, C.; Nabissi, M.; Canesin, G.; Ballarini, P.; Di Spilimbergo, A.; Cardarelli, M.A.; Servi, L.; Mammana, G.; et al. Transient Receptor Potential Vanilloid Type 2 (TRPV2) Expression in Normal Urothelium and in Urothelial Carcinoma of Human Bladder: Correlation with the Pathologic Stage. Eur. Urol. 2008, 54, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Ueda, T.; Shibata, Y.; Ikegami, Y.; Saito, M.; Ishida, Y.; Ugawa, S.; Kohri, K.; Shimada, S. TRPV2 activation induces apoptotic cell death in human T24 bladder cancer cells: A potential therapeutic target for bladder cancer. Urology 2010, 76, 509.e1–509.e7. [Google Scholar] [CrossRef] [PubMed]
- Oulidi, A.; Bokhobza, A.; Gkika, D.; Vanden Abeele, F.; Lehen’kyi, V.; Ouafik, L.; Mauroy, B.; Prevarskaya, N. TRPV2 mediates adrenomedullin stimulation of prostate and urothelial cancer cell adhesion, migration and invasion. PLoS ONE 2013, 8, e64885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monet, M.; Gkika, D.; Lehen’kyi, V.; Pourtier, A.; Vanden Abeele, F.; Bidaux, G.; Juvin, V.; Rassendren, F.; Humez, S.; Prevarsakaya, N. Lysophospholipids stimulate prostate cancer cell migration via TRPV2 channel activation. Biochim. Biophys. Acta 2009, 1793, 3–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monet, M.; Lehen’kyi, V.; Gackiere, F.; Firlej, V.; Vandenberghe, M.; Roudbaraki, M.; Gkika, D.; Pourtier, A.; Bidaux, G.; Slomianny, C.; et al. Role of cationic channel TRPV2 in promoting prostate cancer migration and progression to androgen resistance. Cancer Res. 2010, 70, 3–1235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makker, A.; Goel, M.M. Tumor progression, metastasis, and modulators of epithelial-mesenchymal transition in endometrioid endometrial carcinoma: An update. Endocr. Relat. Cancer 2016, 23, R85–R111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sonoda, K. Molecular biology of gynecological cancer. Oncol. Lett. 2016, 1, 16–22. [Google Scholar] [CrossRef] [Green Version]
- Arora, E.; Masab, M.; Mittar, P.; Jindal, V.; Gupta, S.; Dourado, C. Role of immune checkpoint inhibitors in advanced or recurrent endometrial cancer. Cureus 2018, 10, e2521. [Google Scholar] [CrossRef] [Green Version]
- Ayakannu, T.; Taylor, A.H.; Konje, J.C. Cannabinoid receptor expression in estrogen-dependent and estrogen-independent endometrial cancer. J. Recept. Signal Transduct. Res. 2018, 38, 385–392. [Google Scholar] [CrossRef]
- De Clercq, K.; Held, K.; Van Bree, R.; Meuleman, C.; Peeraer, K.; Tomassetti, C.; Voets, T.; D’Hooghe, T.; Vriens, J. Functional expression of transient receptor potential channels in human endometrial stromal cells during the luteal phase of the menstrual cycle. Hum. Reprod. 2015, 30, 1421–1436. [Google Scholar] [CrossRef] [Green Version]
- Persoons, E.; Hennes, A.; De Clercq, K.; Van Bree, R.; Vriens, G.; O, D.F.; Peterse, D.; Vanhie, A.; Meuleman, C.; Voets, T.; et al. Functional Expression of TRP Ion Channels in Endometrial Stromal Cells of Endometriosis Patients. Int. J. Mol. Sci. 2018, 19, 2467. [Google Scholar] [CrossRef] [Green Version]
- Nabissi, M.; Amant, F.; Gehrig, P. Endometrial Cancer: From Biological to Clinical Approaches; Frontiers Media: Lausanne, Switzerland, 2019. [Google Scholar] [CrossRef]
- Remmerie, M.; Janssens, V. Targeted Therapies in Type II Endometrial Cancers: Too Little, but Not Too Late. Int. J. Mol. Sci. 2018, 19, 2380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, C.R.; Cooper, C.L.; Kurman, R.J.; Goebelsmann, U.; Markland, F.S., Jr. Detection of estrogen receptor in breast and endometrial carcinoma by the immunoperoxidase technique. Cancer 1981, 47, 2634–2640. [Google Scholar] [CrossRef]
- Guida, M.; Ligresti, A.; De Filippis, D.; D’Amico, A.; Petrosino, S.; Cipriano, M.; Bifulco, G.; Simonetti, S.; Orlando, P.; Insabato, L.; et al. The levels of the endocannabinoid receptor CB2 and its ligand 2-arachidonoylglycerol are elevated in endometrial carcinoma. Endocrinology 2010, 151, 921–928. [Google Scholar] [CrossRef] [Green Version]
- Zhou, K.; Zhang, S.S.; Yan, Y.; Zhao, S. Overexpression of transient receptor potential vanilloid 2 is associated with poor prognosis in patients with esophageal squamous cell carcinoma. Med. Oncol. 2014, 31, 7. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Zhu, H.; Yan, Q.; Shen, X.; Lu, X.; Wang, J.; Li, J.; Chen, L. TRPV2-induced Ca(2+)-calcineurin NFAT signaling regulates differentiation of osteoclast in multiple myeloma. Cell Commun. Signal 2018, 16, 1. [Google Scholar] [CrossRef] [Green Version]
- Zoppoli, P.; Calice, G.; Laurino, S.; Ruggieri, V.; La Rocca, F.; La Torre, G.; Ciuffi, M.; Amendola, E.; De Vita, F.; Petrillo, A.; et al. TRPV2 Calcium Channel Gene Expression and Outcomes in Gastric Cancer Patients: A Clinically Relevant Association. J. Clin. Med. 2019, 8, 662. [Google Scholar] [CrossRef] [Green Version]
- Liberati, S.; Morelli, M.B.; Amantini, C.; Farfariello, V.; Santoni, M.; Conti, A.; Nabissi, M.; Cascinu, S.; Santoni, G. Loss of TRPV2 Homeostatic Control of Cell Proliferation Drives Tumor Progression. Cells 2014, 3, 112–128. [Google Scholar] [CrossRef] [Green Version]
- Neumann-Raizel, H.; Shilo, A.; Lev, S.; Mogilevsky, M.; Katz, B.; Shneor, D.; Shaul, Y.D.; Leffler, A.; Gabizon, A.; Karni, R.; et al. 2-APB and CBD-Mediated Targeting of Charged Cytotoxic Compounds Into Tumor Cells Suggests the Involvement of TRPV2 Channels. Front. Pharmacol. 2019, 10, 1198. [Google Scholar] [CrossRef] [Green Version]
- Fraguas-Sánchez, A.I.; Fernández-Carballido, A.; Simancas-Herbada, R.; Martin-Sabroso, C.; Torres-Suárez, A.I. CBD loaded microparticles as a potential formulation to improve paclitaxel and doxorubicin-based chemotherapy in breast cancer. Int. J. Pharm. 2020, 574, 118916. [Google Scholar] [CrossRef]
- López-Valero, I.; Saiz-Ladera, C.; Torres, S.; Hernández-Tiedra, S.; García-Taboada, E.; Rodríguez-Fornés, F.; Barba, M.; Dávila, D.; Salvador-Tormo, N.; Guzmán, M.; et al. Targeting Glioma Initiating Cells with A combined therapy of cannabinoids and temozolomide. Biochem. Pharmacol. 2018, 157, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Torres, S.; Lorente, M.; Rodríguez-Fornés, F.; Hernández-Tiedra, S.; Salazar, M.; García-Taboada, E.; Barcia, J.; Guzmán, M.; Velasco, G. A combined preclinical therapy of cannabinoids and temozolomide against glioma. Mol. Cancer Ther. 2011, 10, 1–103. [Google Scholar] [CrossRef] [Green Version]
- Levy, J.M.M.; Towers, C.G.; Thorburn, A. Targeting autophagy in cancer. Nat. Rev. Cancer. 2017, 17, 528–542. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.; Kim, B.G.; Kim, D.Y.; Kim, B.R.; Kim, J.L.; Park, S.H.; Na, Y.J.; Jo, M.J.; Yun, H.K.; Jeong, Y.A.; et al. Cannabidiol Overcomes Oxaliplatin Resistance by Enhancing NOS3- and SOD2-Induced Autophagy in Human Colorectal Cancer Cells. Cancers 2019, 11, 781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fitzgibbons, P.L.; Dillon, D.A.; Alsabeh, R.; Berman, M.A.; Hayes, D.F.; Hicks, D.G.; Hughes, K.S.; Nofech-Mozes, S. Template for reporting results of biomarker testing of specimens from patients with carcinoma of the breast. Arch. Pathol. Lab. Med. 2014, 138, 595–601. [Google Scholar] [CrossRef] [Green Version]














| TRPV2 | |||
|---|---|---|---|
| High | Moderate | Low | |
| Tumor | 10/53 (18.86%) | 16/53 (30.19%) | 27/53 (50.94%) |
| Serous | 7/29 (24.13%) | 9/29 (31.03%) | 13/29 (45.89%) |
| Clear cell | 1/7 (14.28%) | 1/7 (14.28%) | 5/7 (71.43%) |
| Mixed | 2/17 (11.76%) | 6/17 (35.29%) | 9/17 (52.94%) |
| Peritumoral tissue | 0/5 (0%) | 1/5 (20%) | 4/5 (80%) |
| Normal endometrium | 0/10 (0%) | 4/10 (40%) | 6/10 (60%) |
| FIGO stage | |||
| Stage I–II | 3/20 (15%) | 5/20 (25%) | 12/20 (60%) |
| Serous | 1/10 (10%) | 3/10 (30%) | 6/10 (60%) |
| Clear cell | 0/2 (0%) | 1/2 (50%) | 1/2 (50%) |
| Mixed | 2/8 (25%) | 1/8 (12.5%) | 5/8 (62.5%) |
| Stage III | 3/17 (17.65%) | 6/17 (35.29%) | 8/17 (47.06%) |
| Serous | 3/10 (30%) | 4/10 (40%) | 3/10 (30%) |
| Clear cell | 0/2 (0%) | 0/2 (0%) | 2/2 (100%) |
| Mixed | 0/5 (0%) | 2/5 (40%) | 3/5 (60%) |
| Stage IV | 4/16 (25%) | 5/16 (31.25%) | 7/16 (43.75%) |
| Serous | 4/9 (44.44%) | 2/9 (22.22%) | 3/9 (33.33%) |
| Clear cell | 1/3 (33.33%) | 0/3 (0%) | 2/3 (66.66%) |
| Mixed | 0/4 | 3/4 (75%) | 1/4 (25%) |
| Age | |||
| ≤68 | 4/26 (15.38%) | 6/26 (23.07%) | 16/26 (61.54%) |
| >68 | 6/27 (22.22%) | 10/27 (37.04%) | 11/27 (40.74%) |
| IC50 CBD µg/mL | |||
|---|---|---|---|
| Single Administration | Daily Administration | p Value | |
| Ishikawa | 5.89 ± 0.4 | 3.56 ± 0.2 | ** |
| MFE-280 | 6.33 ± 0.5 | 2.39 ± 0.1 | ** |
| HEC-1A | 23.38 ± 0.8 | 13.16 ± 0.6 | * |
| PCEM002 | 6.58 ± 0.2 | 3.59 ± 0.1 | ** |
| PCEM004a | 20.02 ± 0.7 | 14.01 ± 0.6 | * |
| PCEM004b | 8.29 ± 0.5 | 7.05 ± 0.4 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marinelli, O.; Morelli, M.B.; Annibali, D.; Aguzzi, C.; Zeppa, L.; Tuyaerts, S.; Amantini, C.; Amant, F.; Ferretti, B.; Maggi, F.; et al. The Effects of Cannabidiol and Prognostic Role of TRPV2 in Human Endometrial Cancer. Int. J. Mol. Sci. 2020, 21, 5409. https://doi.org/10.3390/ijms21155409
Marinelli O, Morelli MB, Annibali D, Aguzzi C, Zeppa L, Tuyaerts S, Amantini C, Amant F, Ferretti B, Maggi F, et al. The Effects of Cannabidiol and Prognostic Role of TRPV2 in Human Endometrial Cancer. International Journal of Molecular Sciences. 2020; 21(15):5409. https://doi.org/10.3390/ijms21155409
Chicago/Turabian StyleMarinelli, Oliviero, Maria Beatrice Morelli, Daniela Annibali, Cristina Aguzzi, Laura Zeppa, Sandra Tuyaerts, Consuelo Amantini, Frédéric Amant, Benedetta Ferretti, Federica Maggi, and et al. 2020. "The Effects of Cannabidiol and Prognostic Role of TRPV2 in Human Endometrial Cancer" International Journal of Molecular Sciences 21, no. 15: 5409. https://doi.org/10.3390/ijms21155409
APA StyleMarinelli, O., Morelli, M. B., Annibali, D., Aguzzi, C., Zeppa, L., Tuyaerts, S., Amantini, C., Amant, F., Ferretti, B., Maggi, F., Santoni, G., & Nabissi, M. (2020). The Effects of Cannabidiol and Prognostic Role of TRPV2 in Human Endometrial Cancer. International Journal of Molecular Sciences, 21(15), 5409. https://doi.org/10.3390/ijms21155409