Targeting Chemosensory Ion Channels in Peripheral Swallowing-Related Regions for the Management of Oropharyngeal Dysphagia
Abstract
:1. Introduction
2. Targeting Chemosensory Ion Channels to Improve Swallowing Function
2.1. Targeting Transient Receptor Potential Channels (TRPs)
| Targeting Channels | Agonists and Its Application | Animals | Mode of Application | Effects on Swallowing | Ref. |
|---|---|---|---|---|---|
| TRPV1 | Capsaicin solution (25 μM) into the laryngopharynx and associated laryngeal regions | Rats | Acute |
| [116] |
| Capsaicin solution (10 μM) into the larynx | Guinea pigs | Acute | Capsaicin triggered a greater number of swallowing reflexes compared to saline. | [130] | |
| Capsaicin solution (10 μM) on the vocal folds | Rats | Acute | Capsaicin triggered a considerable number of swallowing reflexes. | [131], [132] | |
| Capsaicin solution (600 nM) into the pharyngolaryngeal region | Rats (a dysphagia model) | Acute | Capsaicin improved the triggering of swallowing reflexes compared to that of distilled water. | [133] | |
| TRPM8 | Menthol solution (50 mM) into the laryngopharynx and associated laryngeal regions | Rats | Acute |
| [116] |
| ASIC3 | Guanidine-4-methylquinazoline (GMQ) solution (0.5 to 10 mM) into the laryngopharynx and associated laryngeal regions | Rats | Acute |
| [117] |
| Agmatine (50 mM to 2 M) solutions into the laryngopharynx and associated laryngeal regions | Rats | Acute |
| [117] | |
| ASICs and TRPV1 | Acetic acid (5 to 30 mM), citric acid (5 to 30 mM) solutions into the pharyngolaryngeal region | Rats | Acute | Acetic acid and citric acid evoked a greater number of swallowing reflexes compared to distilled water. | [134] |
| Citric acid solution (10 mM) into the pharyngolaryngeal region | Rats (a dysphagia model) | Acute | Citric acid solution improved the triggering swallowing reflexes compared to that of distilled water. | [133] |
| Targeting Channels | Agonists and Its Application | Patients/Participants | Mode of Application | Effects on Swallowing | Ref. |
|---|---|---|---|---|---|
| TRPV1 | Capsaicin (1 nM to 1 μM) solution into the pharyngeal region | Aged patients with cerebrovascular diseases or dementia presenting oropharyngeal dysphagia | Acute | Capsaicin solution dose-dependently reduced the latency to trigger a swallow response. | [118] |
| Capsaicinoid (150 μM) containing nectar bolus ingestion | Aged patients presenting oropharyngeal dysphagia | Acute |
| [44] | |
| Capsaicinoid (150 μM) containing nectar bolus ingestion | Aged/stroke/neurodegenerative disease patients presenting oropharyngeal dysphagia | Acute |
| [48] | |
| Capsiate (1–100 nM) into the pharyngeal region | Patients with history of aspiration pneumonia presenting oropharyngeal dysphagia | Acute | Capsiate dose-dependently reduced the latency to trigger a swallow response. | [135] | |
| Capsaicinoid (10 μM) containing nectar bolus ingestion | Aged patients presenting oropharyngeal dysphagia | Chronic (three times/day, before meals for 10 days) |
| [79] | |
| Capsaicin containing pickled cabbage (1.5 μg/10 g) ingestion | Healthy participants | Chronic (before every major meal/day for 20 days) | Latency to trigger a swallow response reduced | [136] | |
| Capsaicin containing lozenges (1.5 μg/lozenge) | Aged patients with cerebrovascular diseases presenting oropharyngeal dysphagia | Chronic (before every major meal/day for 4 weeks) | Latency to trigger a swallow response reduced. | [119] | |
| Capsaicin containing thin film food (0.75 μg/film) ingestion | Aged patients presenting oropharyngeal dysphagia | Chronic (before every major meal/day for 1 week) |
| [113] | |
| Capsaicin (150 μM) containing nectar bolus ingestion along with cold thermal tactile stimulation | Aged patients with history of stroke presenting oropharyngeal dysphagia | Chronic (three times/day, before meals for 3 weeks) | Swallowing function improved assessed by swallowing assessment tools. | [137] | |
| Capsaicinoid (10 μM) containing nectar bolus ingestion | Aged patients presenting oropharyngeal dysphagia | Chronic (three times/day, before meals for 10 days) | The swallowing safety improved evidenced by reduction of the prevalence of aspiration and lowering the score in penetration-aspiration scale. | [114] | |
| Capsaicin (0.5 g of 0.025%) containing ointment into the ear canal | Aged patients presenting oropharyngeal dysphagia | Acute and chronic (once daily for 7 days) | Swallowing function improved. | [138] | |
| TRPM8 | Menthol solution (100 μm to 10 mM) into the pharyngeal region | Aged patients presenting oropharyngeal dysphagia | Acute | Menthol dose-dependently reduced the latency to trigger a swallow response. | [139] |
| Menthol (1 and 10 mM) containing nectar bolus ingestion | Aged/stroke/neurodegenerative diseases patients presenting oropharyngeal dysphagia | Acute |
| [48] | |
| TRPA1 | Cinnamaldehyde (756.6 μM) and zinc (70 μM) containing nectar bolus ingestion | Aged/stroke/neurodegenerative diseases patients presenting oropharyngeal dysphagia | Acute |
| [82] |
| Citral (1.6 mM) containing nectar bolus ingestion | Aged/stroke/neurodegenerative diseases patients presenting oropharyngeal dysphagia | Acute |
| [82] | |
| TRPV1 and TRPA1 | Piperine (150 μM and 1 mM) containing nectar bolus ingestion | Aged/stroke/neurodegenerative diseases patients presenting oropharyngeal dysphagia | Acute |
| [115] |
| Black pepper oil (a volatile compound) (100 μL for 1 min) to the nostrils with a paper stick for inhalation. | Aged patients with cerebrovascular diseases presenting oropharyngeal dysphagia | Acute | Latency to trigger a swallow response for distilled water reduced. | [140] | |
| Piperine (150 μM and 1 mM) containing nectar bolus ingestion | Aged/stroke/neurodegenerative diseases patients presenting oropharyngeal dysphagia | Acute |
| [48] | |
| Black pepper oil (a volatile compound) (100 μL for 1 min) to the nostrils with a paper stick for inhalation. | Aged patients with cerebrovascular diseases presenting oropharyngeal dysphagia | Chronic (three times/day, before meals for 30 days) |
| [140] | |
| Black pepper oil (a volatile compound) (100 μL for 1 min) to the nostrils with a paper stick for inhalation. | Pediatric patients with severe neurological disorders often receiving tube feeding | Chronic (three times/day, before meals for 3 months) |
| [141] | |
| TRPV1, TRPA1 and TRPV3 | Vanillin (a volatile compound), (flow rate 7 L/min for 200 ms) delivered ortho-and retro-nasally | Healthy participants | Acute | The frequency of swallowing for continuous intraoral sweet stimuli (glucose) increased in case of retro-nasal delivery. | [142] |
| TRPA1 and TRPM8 | Citral (1.6 mM) and isopulegol (1.3 mM) containing nectar bolus ingestion | Aged/stroke/neurodegenerative diseases patients presenting oropharyngeal dysphagia | Acute | Upper esophageal opening time during swallowing reduced. | [82] |
| ASICs and TRPV1 | Citric acid (2.7% or 128 mM) containing liquid bolus ingestion | Aged patients with neurological diseases presenting oropharyngeal dysphagia | Acute | Prevalence of aspiration and penetration during swallowing reduced. | [143] |
| Lemon juice containing barium liquid bolus (1:1) ingestion | Patients with strokes and neurological diseases presenting oropharyngeal dysphagia | Acute |
| [49] | |
| Lemon juice containing barium liquid bolus (1:1) ingestion | Healthy participants and head and neck cancer patients | Acute | Pharyngeal transit time reduced. | [144] | |
| Citric acid (80 mM) delivered on the tongue | Healthy participants | Acute |
| [145] | |
| Lemon juice application on the tongue along with nasal inhalation of lemon juice odor | Healthy participants | Acute | Motor evoked potential from the submental muscles increased during volitional swallowing induced by transcranial magnetic stimulation. | [146] | |
| Citric acid solution (20 mM) ingestion | Healthy participants | Acute | Activity of submental muscle during swallowing increased. | [147] | |
| Citric acid solution (2.7% or 128 mM) ingestion | Healthy participants | Acute |
| [148] | |
| Lemon juice (10%) solution ingestion (4 °C before delivery) | Healthy participants and stroke patients with and without oropharyngeal dysphagia | Acute |
| [149] | |
| Lemon juice delivered on tongue | Healthy participants | Acute |
| [150] | |
| Acetic acid (10 and 100 mM) applied on the posterior part of the tongue | Healthy participants | Acute | Latency to trigger swallowing prolonged compared to that of water. | [151] | |
| Citric acid (2.7%) solution ingestion | Healthy participants | Acute | Lingual pressure during swallowing increased. | [152] | |
| Citric acid (10%) solution ingestion | Healthy participants | Acute | Speed of swallowing reduced compared to that of water. | [153] | |
| Citric acid containing gelatin cubes (4.4 g of citric acid in 200 ml of gelatin) chewing and ingestion | Healthy participants | Acute |
| [154] | |
| Lemon water (50%) solution ingestion | Healthy participants | Acute |
| [155] | |
| Lemon juice (a drop of 100% lemon juice in the anterior faucial pillar) + cold mechanical stimuli using a probe (around 8–9 °C) before swallowing of water | Healthy participants | Acute | Latency to trigger swallowing reduced. | [156] | |
| Lemon juice (1:16, mixed with water) ingestion | Healthy participants | Acute | Onset time of activation of the submental and infrahyoid muscles shortened. | [157] |
2.1.1. Targeting TRPV1
Effects of TRPV1 Agonists on Swallowing
2.1.2. Targeting TRPA1
Effects of TRPA1 Agonists on Swallowing
2.1.3. Effects of Dual TRPV1 and TRPA1 Agonists on Swallowing
2.1.4. Targeting TRPM8
Effects of TRPM8 Agonists on Swallowing
2.1.5. Comparison of the Effects of Different TRP Agonists on Swallowing
2.1.6. Stepwise Therapy Using Different TRP Agonists
2.2. Targeting Acid-Sensing Ion Channels (ASICs)
Effects of ASIC Agonists on Swallowing
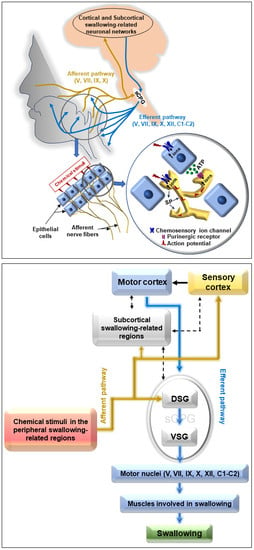
3. Neurophysiological and Molecular Mechanisms of Improving Swallowing Function via the Activation of Chemosensory Ion Channels by Chemical Stimuli
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Goyal, R.K.; Mashimo, H. Physiology of oral, pharyngeal, and esophageal motility. GI Motil. Online 2006, 1–3. [Google Scholar] [CrossRef]
- Miller, A.J. Deglutition. Physiol. Rev. 1982, 62, 129–184. [Google Scholar] [CrossRef] [PubMed]
- Dodds, W.J. The physiology of swallowing. Dysphagia 1989, 3, 171–178. [Google Scholar] [CrossRef]
- Yamamura, K.; Kitagawa, J.; Kurose, M.; Sugino, S.; Takatsuji, H.; Md Mostafeezur, R.; Md Zakir, H.; Yamada, Y. Neural mechanisms of swallowing and effects of taste and other stimuli on swallow initiation. Biol. Pharm. Bull. 2010, 33, 1786–1790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jean, A. Brain stem control of swallowing: Neuronal network and cellular mechanisms. Physiol. Rev. 2001, 81, 929–969. [Google Scholar] [CrossRef] [Green Version]
- Rofes, L.; Arreola, V.; Romea, M.; Palomera, E.; Almirall, J.; Cabré, M.; Serra-Prat, M.; Clavé, P. Pathophysiology of oropharyngeal dysphagia in the frail elderly. Neurogastroenterol. Motil. 2010, 22. [Google Scholar] [CrossRef] [PubMed]
- Carrión, S.; Cabré, M.; Monteis, R.; Roca, M.; Palomera, E.; Serra-Prat, M.; Rofes, L.; Clavé, P. Oropharyngeal dysphagia is a prevalent risk factor for malnutrition in a cohort of older patients admitted with an acute disease to a general hospital. Clin. Nutr. 2015, 34, 436–442. [Google Scholar] [CrossRef]
- Cabre, M.; Serra-Prat, M.; Palomera, E.; Almirall, J.; Pallares, R.; Clavé, P. Prevalence and prognostic implications of dysphagia in elderly patients with pneumonia. Age Ageing 2009, 39, 39–45. [Google Scholar] [CrossRef] [Green Version]
- Ebihara, S.; Sekiya, H.; Miyagi, M.; Ebihara, T.; Okazaki, T. Dysphagia, dystussia, and aspiration pneumonia in elderly people. J. Thorac. Dis. 2016, 8, 632–639. [Google Scholar] [CrossRef] [Green Version]
- Cabré, M.; Serra-Prat, M.; Force, L.; Almirall, J.; Palomera, E.; Clavé, P. Oropharyngeal dysphagia is a risk factor for readmission for pneumonia in the very elderly persons: Observational prospective study. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2014, 69A, 330–337. [Google Scholar] [CrossRef] [Green Version]
- Manabe, T.; Teramoto, S.; Tamiya, N.; Okochi, J.; Hizawa, N. Risk Factors for Aspiration Pneumonia in Older Adults. PLoS ONE 2015, 10, e0140060. [Google Scholar] [CrossRef] [PubMed]
- Clavé, P.; Rofes, L.; Arreola, V.; Almirall, J.; Cabré, M.; Campins, L.; García-Peris, P.; Speyer, R. Diagnosis and management of oropharyngeal dysphagia and its nutritional and respiratory complications in the elderly. Gastroenterol. Res. Pract. 2011, 2011, 13. [Google Scholar]
- Koidou, I.; Kollias, N.; Sdravou, K.; Grouios, G. Dysphagia: A Short Review of the Current State. Educ. Gerontol. 2013, 39, 812–827. [Google Scholar] [CrossRef]
- Seaman, W.B. Pharyngeal and Upper Esophageal Dysphagia. JAMA J. Am. Med. Assoc. 1976, 235, 2643–2646. [Google Scholar] [CrossRef]
- Spieker, M.R. Evaluating dysphagia. Am. Fam. Phys. 2000, 61, 3639–3648. [Google Scholar]
- Clavé, P.; Shaker, R. Dysphagia: Current reality and scope of the problem. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 259–270. [Google Scholar] [CrossRef]
- Jaffer, N.M.; Ng, E.; Au, F.W.F.; Steele, C.M. Fluoroscopic evaluation of oropharyngeal dysphagia: Anatomic, technical, and common etiologic factors. Am. J. Roentgenol. 2015, 204, 49–58. [Google Scholar] [CrossRef] [Green Version]
- Matsuo, K.; Palmer, J.B. Anatomy and Physiology of Feeding and Swallowing: Normal and Abnormal. Phys. Med. Rehabil. Clin. N. Am. 2008, 19, 691–707. [Google Scholar] [CrossRef] [Green Version]
- Clavé, P.; De Kraa, M.; Arreola, V.; Girvent, M.; Farré, R.; Palomera, E.; Serra-Prat, M. The effect of bolus viscosity on swallowing function in neurogenic dysphagia. Aliment. Pharmacol. Ther. 2006, 24, 1385–1394. [Google Scholar] [CrossRef]
- Lazarus, C.L. Effects of chemoradiotherapy on voice and swallowing. Curr. Opin. Otolaryngol. Head Neck Surg. 2009, 17, 172–178. [Google Scholar] [CrossRef]
- Newman, R.; Vilardell, N.; Clavé, P.; Speyer, R. Effect of Bolus Viscosity on the Safety and Efficacy of Swallowing and the Kinematics of the Swallow Response in Patients with Oropharyngeal Dysphagia: White Paper by the European Society for Swallowing Disorders (ESSD). Dysphagia 2016, 31, 232–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cook, I.J. Oropharyngeal Dysphagia. Gastroenterol. Clin. N. Am. 2009, 38, 411–431. [Google Scholar] [CrossRef] [PubMed]
- Bulat, R.S.; Orlando, R.C. Oropharyngeal dysphagia. Curr. Treat. Options Gastroenterol. 2005, 8, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Shaker, R. Oropharyngeal Dysphagia. Gastroenterol. Hepatol. 2006, 2, 633–634. [Google Scholar]
- Daniels, S. Neurological disorders affecting oral, pharyngeal swallowing. GI Motil. Online 2006, 2210. [Google Scholar] [CrossRef]
- de Souza Oliveira, A.R.; de Sousa Costa, A.G.; Morais, H.C.C.; Cavalcante, T.F.; de Oliveira Lopes, M.V.; de Araujo, T.L. Clinical factors predicting risk for aspiration and respiratory aspiration among patients with Stroke. Rev. Lat. Am. Enferm. 2015, 23, 216–224. [Google Scholar] [CrossRef]
- Kreuzer, S.H.; Schima, W.; Schober, E.; Pokieser, P.; Kofler, G.; Lechner, G.; Denk, D.M. Complications after laryngeal surgery: Videofluoroscopic evaluation of 120 patients. Clin. Radiol. 2000, 55, 775–781. [Google Scholar] [CrossRef]
- Wirth, R.; Dziewas, R.; Beck, A.M.; Clavé, P.; Hamdy, S.; Heppner, H.J.; Langmore, S.; Leischker, A.H.; Martino, R.; Pluschinski, P.; et al. Oropharyngeal dysphagia in older persons—From pathophysiology to adequate intervention: A review and summary of an international expert meeting. Clin. Interv. Aging 2016, 11, 189–208. [Google Scholar] [CrossRef] [Green Version]
- Ortega, O.; Cabre, M.; Clave, P. Oropharyngeal dysphagia: Aetiology and effects of ageing. J. Gastroenterol. Hepatol. Res. 2014, 3, 1049–1054. [Google Scholar] [CrossRef]
- Robbins, J.; Bridges, A.D.; Taylor, A. Oral, pharyngeal and esophageal motor function in aging. GI Motil. Online 2006, 1–21. [Google Scholar] [CrossRef]
- Espinosa-Val, C.; Martín-Martínez, A.; Graupera, M.; Arias, O.; Elvira, A.; Cabré, M.; Palomera, E.; Bolívar-Prados, M.; Clavé, P.; Ortega, O. Prevalence, risk factors, and complications of oropharyngeal dysphagia in older patients with dementia. Nutrients 2020, 12, 863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, L.C.; Wu, S.C.; Chen, H.S.; Wang, T.G.; Chen, M.Y. Prevalence of impaired swallowing in institutionalized older people in Taiwan. J. Am. Geriatr. Soc. 2002, 50, 1118–1123. [Google Scholar] [CrossRef] [PubMed]
- Bloem, B.R.; Lagaay, A.M.; Van Beek, W.; Haan, J.; Roos, R.A.C.; Wintzen, A.R. Prevalence of subjective dysphagia in community residents aged over 87. Br. Med. J. 1990, 300, 721–722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serra-Prat, M.; Hinojosa, G.; Lõpez, D.; Juan, M.; Fabré, E.; Voss, D.S.; Calvo, M.; Marta, V.; Ribõ, L.; Palomera, E.; et al. Prevalence of oropharyngeal dysphagia and impaired safety and efficacy of swallow in independently living older persons. J. Am. Geriatr. Soc. 2011, 59, 186–187. [Google Scholar] [CrossRef] [PubMed]
- Almirall, J.; Rofes, L.; Serra-Prat, M.; Icart, R.; Palomera, E.; Arreola, V.; Clavé, P. Oropharyngeal dysphagia is a risk factor for community-acquired pneumonia in the elderly. Eur. Respir. J. 2013, 41, 923–926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wirth, R.; Dziewas, R. Dysphagia and pharmacotherapy in older adults. Curr. Opin. Clin. Nutr. Metab. Care 2019, 22, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Dziewas, R.; Beck, A.M.; Clave, P.; Hamdy, S.; Heppner, H.J.; Langmore, S.E.; Leischker, A.; Martino, R.; Pluschinski, P.; Roesler, A.; et al. Recognizing the Importance of Dysphagia: Stumbling Blocks and Stepping Stones in the Twenty-First Century. Dysphagia 2017, 32, 78–82. [Google Scholar] [CrossRef] [Green Version]
- Cohen, D.L.; Roffe, C.; Beavan, J.; Blackett, B.; Fairfield, C.A.; Hamdy, S.; Havard, D.; McFarlane, M.; McLauglin, C.; Randall, M.; et al. Post-stroke dysphagia: A review and design considerations for future trials. Int. J. Stroke 2016, 11, 399–411. [Google Scholar] [CrossRef]
- Cabib, C.; Ortega, O.; Kumru, H.; Palomeras, E.; Vilardell, N.; Alvarez-Berdugo, D.; Muriana, D.; Rofes, L.; Terré, R.; Mearin, F.; et al. Neurorehabilitation strategies for poststroke oropharyngeal dysphagia: From compensation to the recovery of swallowing function. Ann. N. Y. Acad. Sci. 2016, 1380, 121–138. [Google Scholar] [CrossRef]
- Ortega, O.; Martín, A.; Clavé, P. Diagnosis and Management of Oropharyngeal Dysphagia Among Older Persons, State of the Art. J. Am. Med. Dir. Assoc. 2017, 18, 576–582. [Google Scholar] [CrossRef]
- Martino, R.; McCulloch, T. Therapeutic intervention in oropharyngeal dysphagia. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 665–679. [Google Scholar] [CrossRef] [PubMed]
- Langmore, S.E.; Pisegna, J.M. Efficacy of exercises to rehabilitate dysphagia: A critique of the literature. Int. J. Speech. Lang. Pathol. 2015, 17, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Speyer, R.; Baijens, L.; Heijnen, M.; Zwijnenberg, I. Effects of therapy in oropharyngeal dysphagia by speech and language therapists: A systematic review. Dysphagia 2010, 25, 40–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rofes, L.; Arreola, V.; Martin, A.; Clavé, P. Natural capsaicinoids improve swallow response in older patients with oropharyngeal dysphagia. Gut 2013, 62, 1280–1287. [Google Scholar] [CrossRef] [PubMed]
- Bisch, E.M.; Logemann, J.A.; Rademaker, A.W.; Kahrilas, P.J.; Lazarus, C.L. Pharyngeal effects of bolus volume, viscosity, and temperature in patients with dysphagia resulting from neurologic impairment and in normal subjects. J. Speech Hear. Res. 1994, 37, 1041–1049. [Google Scholar] [CrossRef] [Green Version]
- Rofes, L.; Arreola, V.; Mukherjee, R.; Swanson, J.; Clavé, P. The effects of a xanthan gum-based thickener on the swallowing function of patients with dysphagia. Aliment. Pharmacol. Ther. 2014, 39, 1169–1179. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, N.; Kotz, T.; Shapiro, J. The effect of bolus consistency on dysphagia in unilateral vocal cord paralysis. Otolaryngol. Head Neck Surg. 2003, 129, 632–636. [Google Scholar] [CrossRef]
- Alvarez-Berdugo, D.; Rofes, L.; Arreola, V.; Martin, A.; Molina, L.; Clavé, P. A comparative study on the therapeutic effect of TRPV1, TRPA1, and TRPM8 agonists on swallowing dysfunction associated with aging and neurological diseases. Neurogastroenterol. Motil. 2018, 30. [Google Scholar] [CrossRef]
- Logemann, J.A.; Pauloski, B.R.; Colangelo, L.; Lazarus, C.; Fujiu, M.; Kahrilas, P.J. Effects of a sour bolus on oropharyngeal swallowing measures in patients with neurogenic dysphagia. J. Speech Hear. Res. 1995, 38, 556–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mistry, S.; Michou, E.; Vasant, D.H.; Hamdy, S. Direct and Indirect Therapy: Neurostimulation for the Treatment of Dysphagia After Stroke; Springer: Heidelberg, Germany, 2011; pp. 519–538. [Google Scholar]
- Logemann, J.A. Treatment of Oral and Pharyngeal Dysphagia. Phys. Med. Rehabil. Clin. N. Am. 2008, 19, 803–816. [Google Scholar] [CrossRef] [PubMed]
- Ashford, J.; McCabe, D.; Wheeler-Hegland, K.; Frymark, T.; Mullen, R.; Musson, N.; Schooling, T.; Hammond, C.S. Evidence-based systematic review: Oropharyngeal dysphagia behavioral treatments. Part III—Impact of dysphagia treatments on populations with neurological disorders. J. Rehabil. Res. Dev. 2009, 46, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Bath, P.M.; Lee, H.S.; Everton, L.F. Swallowing therapy for dysphagia in acute and subacute stroke. Cochrane Database Syst. Rev. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Berdugo, D.; Tomsen, N.; Clavé, P. Sensory stimulation treatments for oropharyngeal dysphagia. In Medical Radiology; Springer: Berlin, Germany, 2019; pp. 763–779. [Google Scholar]
- Wang, Z.; Song, W.Q.; Wang, L. Application of noninvasive brain stimulation for post-stroke dysphagia rehabilitation. Kaohsiung J. Med. Sci. 2017, 33, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Simons, A.; Hamdy, S. The Use of Brain Stimulation in Dysphagia Management. Dysphagia 2017, 32, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Pisegna, J.M.; Kaneoka, A.; Pearson, W.G.; Kumar, S.; Langmore, S.E. Effects of non-invasive brain stimulation on post-stroke dysphagia: A systematic review and meta-analysis of randomized controlled trials. Clin. Neurophysiol. 2016, 127, 956–968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.N.; Pyun, S.B.; Kim, H.J.; Ahn, H.S.; Rhyu, B.J. Effectiveness of Non-invasive Brain Stimulation in Dysphagia Subsequent to Stroke: A Systemic Review and Meta-analysis. Dysphagia 2015, 30, 383–391. [Google Scholar] [CrossRef]
- Fraser, C.; Power, M.; Hamdy, S.; Rothwell, J.; Hobday, D.; Hollander, I.; Tyrell, P.; Hobson, A.; Williams, S.; Thompson, D. Driving plasticity in human adult motor cortex is associated with improved motor function after brain injury. Neuron 2002, 34, 831–840. [Google Scholar] [CrossRef] [Green Version]
- Khedr, E.M.; Abo-Elfetoh, N.; Rothwell, J.C. Treatment of post-stroke dysphagia with repetitive transcranial magnetic stimulation. Acta Neurol. Scand. 2009, 119, 155–161. [Google Scholar] [CrossRef]
- Papadopoulou, S.L.; Ploumis, A.; Exarchakos, G.; Theodorou, S.; Beris, A.; Fotopoulos, A. Versatility of repetitive transcranial magnetic stimulation in the treatment of poststroke dysphagia. J. Neurosci. Rural Pract. 2018, 9, 391–396. [Google Scholar] [CrossRef]
- Doeltgen, S.H.; Huckabee, M.L. Swallowing neurorehabilitation: From the research laboratory to routine clinical application. Arch. Phys. Med. Rehabil. 2012, 93, 207–213. [Google Scholar] [CrossRef] [Green Version]
- Rossi, S.; Hallett, M.; Rossini, P.M.; Pascual-Leone, A.; Avanzini, G.; Bestmann, S.; Berardelli, A.; Brewer, C.; Canli, T.; Cantello, R.; et al. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin. Neurophysiol. 2009, 120, 2008–2039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rofes, L.; Cola, P.C.; Clave, P. The effects of sensory stimulation on neurogenic oropharyngeal dysphagia. J. Gastroenterol. Hepatol. Res. 2014, 3, 1066–1072. [Google Scholar] [CrossRef]
- Lowell, S.Y.; Poletto, C.J.; Knorr-Chung, B.R.; Reynolds, R.C.; Simonyan, K.; Ludlow, C.L. Sensory stimulation activates both motor and sensory components of the swallowing system. Neuroimage 2008, 42, 285–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steele, C.M.; Miller, A.J. Sensory input pathways and mechanisms in swallowing: A review. Dysphagia 2010, 25, 323–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, A.J. Significance of sensory inflow to the swallowing reflex. Brain Res. 1972, 43, 147–159. [Google Scholar] [CrossRef]
- Jean, A.; Car, A. Inputs to the swallowing medullary neurons from the peripheral afferent fibers and the swallowing cortical area. Brain Res. 1979, 178, 567–572. [Google Scholar] [CrossRef]
- Ertekin, C.; Kiylioglu, N.; Tarlaci, S.; Keskin, A.; Aydogdu, I. Effect of mucosal anaesthesia on oropharyngeal swallowing. Neurogastroenterol. Motil. 2000, 12, 567–572. [Google Scholar] [CrossRef]
- Teismann, I.K.; Steinstraeter, O.; Stoeckigt, K.; Suntrup, S.; Wollbrink, A.; Pantev, C.; Dziewas, R. Functional oropharyngeal sensory disruption interferes with the cortical control of swallowing. BMC Neurosci. 2007, 8. [Google Scholar] [CrossRef] [Green Version]
- Fraser, C.; Rothwell, J.; Power, M.; Hobson, A.; Thompson, D.; Hamdy, S. Differential changes in human pharyngoesophageal motor excitability induced by swallowing, pharyngeal stimulation, and anesthesia. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 285. [Google Scholar] [CrossRef] [Green Version]
- Jafari, S.; Prince, R.A.; Kim, D.Y.; Paydarfar, D. Sensory regulation of swallowing and airway protection: A role for the internal superior laryngeal nerve in humans. J. Physiol. 2003, 550, 287–304. [Google Scholar] [CrossRef]
- Sulica, L.; Hembree, A.; Blitzer, A. Swallowing and sensation: Evaluation of deglutition in the anesthetized larynx. Ann. Otol. Rhinol. Laryngol. 2002, 111, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Tiago, R.; Pontes, P.; do Brasil, O.C. Age-related changes in human laryngeal nerves. Otolaryngol. Head Neck Surg. 2007, 136, 747–751. [Google Scholar] [CrossRef] [PubMed]
- Mortelliti, A.J.; Malmgren, L.T.; Gacek, R.R. Ultrastructural Changes With Age in the Human Superior Laryngeal Nerve. Arch. Otolaryngol. Neck Surg. 1990, 116, 1062–1069. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.H.; Diamond, B.; Aviv, J.E.; Sacco, R.L.; Keen, M.S.; Zagar, D.; Blitzer, A. Supraglottic and pharyngeal sensory abnormalities in stroke patients with dysphagia. Ann. Otol. Rhinol. Laryngol. 1996, 105, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.H.; Diamond, B.; Aviv, J.E.; Jones, M.E.; Keen, M.S.; Wee, T.A.; Blitzer, A. Age-Related changes in pharyngeal and supraglottic sensation. Ann. Otol. Rhinol. Laryngol. 1994, 103, 749–752. [Google Scholar] [CrossRef]
- Rofes, L.; Ortega, O.; Vilardell, N.; Mundet, L.; Clavé, P. Spatiotemporal characteristics of the pharyngeal event-related potential in healthy subjects and older patients with oropharyngeal dysfunction. Neurogastroenterol. Motil. 2017, 29. [Google Scholar] [CrossRef]
- Tomsen, N.; Ortega, O.; Rofes, L.; Arreola, V.; Martin, A.; Mundet, L.; Clavé, P. Acute and subacute effects of oropharyngeal sensory stimulation with TRPV1 agonists in older patients with oropharyngeal dysphagia: A biomechanical and neurophysiological randomized pilot study. Therap. Adv. Gastroenterol. 2019, 12. [Google Scholar] [CrossRef] [Green Version]
- Shaw, D.W.; Cook, I.J.; Gabb, M.; Holloway, R.H.; Simula, M.E.; Panagopoulos, V.; Dent, J. Influence of normal aging on oral-pharyngeal and upper esophageal sphincter function during swallowing. Am. J. Physiol. Gastrointest. Liver Physiol. 1995, 268. [Google Scholar] [CrossRef]
- Robbins, J.; Hamilton, J.W.; Lof, G.L.; Kempster, G.B. Oropharyngeal swallowing in normal adults of different ages. Gastroenterology 1992, 103, 823–829. [Google Scholar] [CrossRef]
- Tomsen, N.; Alvarez-Berdugo, D.; Rofes, L.; Ortega, O.; Arreola, V.; Nascimento, W.; Martin, A.; Cabib, C.; Bolivar-Prados, M.; Mundet, L.; et al. A randomized clinical trial on the acute therapeutic effect of TRPA1 and TRPM8 agonists in patients with oropharyngeal dysphagia. Neurogastroenterol. Motil. 2020, 32. [Google Scholar] [CrossRef]
- Humbert, I.A.; Fitzgerald, M.E.; McLaren, D.G.; Johnson, S.; Porcaro, E.; Kosmatka, K.; Hind, J.; Robbins, J.A. Neurophysiology of swallowing: Effects of age and bolus type. Neuroimage 2009, 44, 982–991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aviv, J.E. Effects of Aging on Sensitivity of the Pharyngeal and Supraglottic Areas. Am. J. Med. 1997, 103, 74S–76S. [Google Scholar] [CrossRef]
- Onofri, S.M.M.; Cola, P.C.; Berti, L.C.; Da Silva, R.G.; Dantas, R.O. Correlation between laryngeal sensitivity and penetration/aspiration after stroke. Dysphagia 2014, 29, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Hammer, M.J.; Murphy, C.A.; Abrams, T.M. Airway somatosensory deficits and dysphagia in parkinson’s disease. J. Parkinsons. Dis. 2013, 3, 39–44. [Google Scholar] [CrossRef] [Green Version]
- Theurer, J.A.; Bihari, F.; Barr, A.M.; Martin, R.E. Oropharyngeal stimulation with air-pulse trains increases swallowing frequency in healthy adults. Dysphagia 2005, 20, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Theurer, J.A.; Czachorowski, K.A.; Martin, L.P.; Martin, R.E. Effects of oropharyngeal air-pulse stimulation on swallowing in healthy older adults. Dysphagia 2009, 24, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Selçuk, B.; Uysal, H.; Aydogdu, I.; Akyuz, M.; Ertekin, C. Effect of temperature on electrophysiological parameters of swallowing. J. Rehabil. Res. Dev. 2007, 44, 373–380. [Google Scholar] [CrossRef]
- de Lama Lazzara, G.; Lazarus, C.; Logemann, J.A. Impact of thermal stimulation on the triggering of the swallowing reflex. Dysphagia 1986, 1, 73–77. [Google Scholar] [CrossRef]
- Ali, G.N.; Laundl, T.M.; Wallace, K.L.; DeCarle, D.J.; Cook, I.J.S. Influence of cold stimulation on the normal pharyngeal swallow response. Dysphagia 1996, 11, 2–8. [Google Scholar] [CrossRef]
- Teismann, I.K.; Steinsträter, O.; Warnecke, T.; Suntrup, S.; Ringelstein, E.B.; Pantev, C.; Dziewas, R. Tactile thermal oral stimulation increases the cortical representation of swallowing. BMC Neurosci. 2009, 10. [Google Scholar] [CrossRef] [Green Version]
- Kaatzke-McDonald, M.N.; Post, E.; Davis, P.J. The effects of cold, touch, and chemical stimulation of the anterior faucial pillar on human swallowing. Dysphagia 1996, 11, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Miyaoka, Y.; Haishima, K.; Takagi, M.; Haishima, H.; Asari, J.; Yamada, Y. Influences of thermal and gustatory characteristics on sensory and motor aspects of swallowing. Dysphagia 2006, 21, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Knauer, C.M.; Castell, J.A.; Dalton, C.B.; Nowak, L.; Castell, D.O. Pharyngeal/upper esophageal sphincter pressure dynamics in humans. Effects of pharmacologic agents and thermal stimulation. Dig. Dis. Sci. 1990, 35, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Rosenbek, J.C.; Robbins, J.; Fishback, B.; Levine, R.L. Effects of thermal application on dysphagia after stroke. J. Speech Hear. Res. 1991, 34, 1257–1268. [Google Scholar] [CrossRef] [PubMed]
- Vasant, D.H.; Michou, E.; O’Leary, N.; Vail, A.; Mistry, S.; Hamdy, S. Pharyngeal Electrical Stimulation in Dysphagia Poststroke: A Prospective, Randomized Single-Blinded Interventional Study. Neurorehabil. Neural Repair 2016, 30, 866–875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michou, E.; Mistry, S.; Jefferson, S.; Tyrrell, P.; Hamdy, S. Characterizing the mechanisms of central and peripheral forms of neurostimulation in chronic dysphagic stroke patients. Brain Stimul. 2014, 7, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Takeishi, R.; Magara, J.; Watanabe, M.; Tsujimura, T.; Hayashi, H.; Hori, K.; Inoue, M. Effects of pharyngeal electrical stimulation on swallowing performance. PLoS ONE 2018, 13, e0190608. [Google Scholar] [CrossRef]
- Restivo, D.A.; Hamdy, S. Pharyngeal electrical stimulation device for the treatment of neurogenic dysphagia: Technology update. Med. Devices Evid. Res. 2018, 11, 21–26. [Google Scholar] [CrossRef] [Green Version]
- Suntrup, S.; Teismann, I.; Wollbrink, A.; Winkels, M.; Warnecke, T.; Pantev, C.; Dziewas, R. Pharyngeal electrical stimulation can modulate swallowing in cortical processing and behavior Magnetoencephalographic evidence. Neuroimage 2015, 104, 117–124. [Google Scholar] [CrossRef]
- Takatsuji, H.; Zakir, H.M.; Mostafeezur, R.M.; Saito, I.; Yamada, Y.; Yamamura, K.; Kitagawa, J. Induction of the swallowing reflex by electrical stimulation of the posterior oropharyngeal region in awake humans. Dysphagia 2012, 27, 473–480. [Google Scholar] [CrossRef]
- Jayasekeran, V.; Singh, S.; Tyrrell, P.; Michou, E.; Jefferson, S.; Mistry, S.; Gamble, E.; Rothwell, J.; Thompson, D.; Hamdy, S. Adjunctive Functional Pharyngeal Electrical Stimulation Reverses Swallowing Disability After Brain Lesions. Gastroenterology 2010, 138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rofes, L.; Arreola, V.; López, I.; Martin, A.; Sebastián, M.; Ciurana, A.; Clavé, P. Effect of surface sensory and motor electrical stimulation on chronic poststroke oropharyngeal dysfunction. Neurogastroenterol. Motil. 2013, 25. [Google Scholar] [CrossRef] [PubMed]
- Carnaby-Mann, G.D.; Crary, M.A. Examining the evidence on neuromuscular electrical stimulation for swallowing: A meta-analysis. Arch. Otolaryngol. Head Neck Surg. 2007, 133, 564–571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Tao, T.; Zhang, Z.B.; Zhu, X.; Fan, W.G.; Pu, L.J.; Chu, L.; Yue, S.W. Effectiveness of neuromuscular electrical stimulation on patients with dysphagia with medullary infarction. Arch. Phys. Med. Rehabil. 2016, 97, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.W.; Chang, K.H.; Chen, H.C.; Liang, W.M.; Wang, Y.H.; Lin, Y.N. The effects of surface neuromuscular electrical stimulation on post-stroke dysphagia: A systemic review and meta-analysis. Clin. Rehabil. 2016, 30, 24–35. [Google Scholar] [CrossRef]
- Tan, C.; Liu, Y.; Li, W.; Liu, J.; Chen, L. Transcutaneous neuromuscular electrical stimulation can improve swallowing function in patients with dysphagia caused by non-stroke diseases: A meta-analysis. J. Oral Rehabil. 2013, 40, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Christiaanse, M.E.; Mabe, B.; Russell, G.; Simeone, T.L.; Fortunato, J.; Rubin, B. Neuromuscular electrical stimulation is no more effective than usual care for the treatment of primary dysphagia in children. Pediatr. Pulmonol. 2011, 46, 559–565. [Google Scholar] [CrossRef]
- Ludlow, C.L.; Humbert, I.; Saxon, K.; Poletto, C.; Sonies, B.; Crujido, L. Effects of surface electrical stimulation both at rest and during swallowing in chronic pharyngeal dysphagia. Dysphagia 2007, 22, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Baijens, L.W.J.; Speyer, R.; Passos, V.L.; Pilz, W.; Van Der Kruis, J.; Haarmans, S.; Desjardins-Rombouts, C. Surface electrical stimulation in dysphagic Parkinson patients: A randomized clinical trial. Laryngoscope 2013, 123. [Google Scholar] [CrossRef]
- Gallas, S.; Marie, J.P.; Leroi, A.M.; Verin, E. Sensory transcutaneous electrical stimulation improves post-stroke dysphagic patients. Dysphagia 2010, 25, 291–297. [Google Scholar] [CrossRef]
- Nakato, R.; Manabe, N.; Shimizu, S.; Hanayama, K.; Shiotani, A.; Hata, J.; Haruma, K. Effects of Capsaicin on Older Patients with Oropharyngeal Dysphagia: A Double-Blind, Placebo-Controlled, Crossover Study. Digestion 2017, 95, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Ortega, O.; Rofes, L.; Martin, A.; Arreola, V.; López, I.; Clavé, P. A Comparative Study Between Two Sensory Stimulation Strategies After Two Weeks Treatment on Older Patients with Oropharyngeal Dysphagia. Dysphagia 2016, 31, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Rofes, L.; Arreola, V.; Martin, A.; Clavé, P. Effect of oral piperine on the swallow response of patients with oropharyngeal dysphagia. J. Gastroenterol. 2014, 49, 1517–1523. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.Z.; Ando, H.; Unno, S.; Masuda, Y.; Kitagawa, J. Activation of TRPV1 and TRPM8 channels in the larynx and associated laryngopharyngeal regions facilitates the swallowing reflex. Int. J. Mol. Sci. 2018, 19, 4113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hossain, M.Z.; Ando, H.; Unno, S.; Nakamoto, T.; Kitagawa, J. Functional involvement of acid-sensing ion channel 3 in the swallowing reflex in rats. Neurogastroenterol. Motil. 2020, 32. [Google Scholar] [CrossRef]
- Ebihara, T.; Sekizawa, K.; Nakazawa, H.; Sasaki, H. Capsaicin and swallowing reflex. Lancet 1993, 341, 432. [Google Scholar] [CrossRef]
- Ebihara, T.; Takahashi, H.; Ebihara, S.; Okazaki, T.; Sasaki, T.; Watando, A.; Nemoto, M.; Sasaki, H. Capsaicin troche for swallowing dysfunction in older people. J. Am. Geriatr. Soc. 2005, 53, 824–828. [Google Scholar] [CrossRef]
- Bradley, R.M.; Stedman, H.M.; Mistretta, C.M. Superior laryngeal nerve response patterns to chemical stimulation of sheep epiglottis. Brain Res. 1983, 276, 81–93. [Google Scholar] [CrossRef] [Green Version]
- Smith, D.V.; Hanamori, T. Organization of gustatory sensitivities in hamster superior laryngeal nerve fibers. J. Neurophysiol. 1991, 65, 1098–1114. [Google Scholar] [CrossRef]
- Ohkuri, T.; Horio, N.; Stratford, J.M.; Finger, T.E.; Ninomiya, Y. Residual chemoresponsiveness to acids in the superior laryngeal nerve in “taste-blind” (p2x2/p2x3 double-ko) mice. Chem. Senses 2012, 37, 523–532. [Google Scholar] [CrossRef]
- Kitagawa, J.; Takahashi, Y.; Matsumoto, S.; Shingai, T. Response properties of the pharyngeal branch of the glossopharyngeal nerve for umami taste in mice and rats. Neurosci. Lett. 2007, 417, 42–45. [Google Scholar] [CrossRef] [PubMed]
- Sweazey, R.D.; Bradley, R.M. Responses of lamb nucleus of the solitary tract neurons to chemical stimulation of the epiglottis. Brain Res. 1988, 439, 195–210. [Google Scholar] [CrossRef] [Green Version]
- Nilius, B.; Szallasi, A. Transient receptor potential channels as drug targets: From the science of basic research to the art of medicine. Pharmacol. Rev. 2014, 66, 676–814. [Google Scholar] [CrossRef] [PubMed]
- Szallasi, A.; Cortright, D.N.; Blum, C.A.; Eid, S.R. The vanilloid receptor TRPV1: 10 years from channel cloning to antagonist proof-of-concept. Nat. Rev. Drug Discov. 2007, 6, 357–372. [Google Scholar] [CrossRef] [PubMed]
- Clapham, D.E. TRP channels as cellular sensors. Nature 2003, 426, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.Z.; Bakri, M.M.; Yahya, F.; Ando, H.; Unno, S.; Kitagawa, J. The role of transient receptor potential (TRP) channels in the transduction of dental pain. Int. J. Mol. Sci. 2019, 20, 526. [Google Scholar] [CrossRef] [Green Version]
- Nilius, B. TRP channels in disease. Biochim. Biophys. Acta Mol. Basis Dis. 2007, 1772, 805–812. [Google Scholar] [CrossRef] [Green Version]
- Tsujimura, T.; Udemgba, C.; Inoue, M.; Canning, B.J. Laryngeal and tracheal afferent nerve stimulation evokes swallowing in anaesthetized guinea pigs. J. Physiol. 2013, 591, 4667–4679. [Google Scholar] [CrossRef]
- Tsujimura, T.; Sakai, S.; Suzuki, T.; Ujihara, I.; Tsuji, K.; Magara, J.; Canning, B.J.; Inoue, M. Central inhibition of initiation of swallowing by systemic administration of diazepam and baclofen in anaesthetized rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 312, G498–G507. [Google Scholar] [CrossRef] [Green Version]
- Tsujimura, T.; Ueha, R.; Yoshihara, M.; Takei, E.; Nagoya, K.; Shiraishi, N.; Magara, J.; Inoue, M. Involvement of the epithelial sodium channel in initiation of mechanically evoked swallows in anaesthetized rats. J. Physiol. 2019, 597, 2949–2963. [Google Scholar] [CrossRef]
- Sugiyama, N.; Nishiyama, E.; Nishikawa, Y.; Sasamura, T.; Nakade, S.; Okawa, K.; Nagasawa, T.; Yuki, A. A novel animal model of dysphagia following stroke. Dysphagia 2014, 29, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Kajii, Y.; Shingai, T.; Kitagawa, J.I.; Takahashi, Y.; Taguchi, Y.; Noda, T.; Yamada, Y. Sour taste stimulation facilitates reflex swallowing from the pharynx and larynx in the rat. Physiol. Behav. 2002, 77, 321–325. [Google Scholar] [CrossRef]
- Yamasaki, M.; Ebihara, S.; Ebihara, T.; Yamanda, S.; Arai, H.; Kohzuki, M. Effects of capsiate on the triggering of the swallowing reflex in elderly patients with aspiration pneumonia. Geriatr. Gerontol. Int. 2010, 10, 107–109. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Shutoh, N.; Tonai, M.; Ogata, N. The Effect of Capsaicin-Containing Food on the Swallowing Response. Dysphagia 2016, 31, 146–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Wu, L.; Fang, Q.; Shen, M.; Zhang, L.; Liu, X. Effects of capsaicin on swallowing function in stroke patients with dysphagia: A randomized controlled trial. J. Stroke Cerebrovasc. Dis. 2019, 28, 1744–1751. [Google Scholar] [CrossRef] [PubMed]
- Kondo, E.; Jinnouchi, O.; Ohnishi, H.; Kawata, I.; Nakano, S.; Goda, M.; Kitamura, Y.; Abe, K.; Hoshikawa, H.; Okamoto, H.; et al. Effects of aural stimulation with capsaicin ointment on swallowing function in elderly patients with non-obstructive dysphagia. Clin. Interv. Aging 2014, 9, 1661–1667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebihara, T.; Ebihara, S.; Watando, A.; Okazaki, T.; Asada, M.; Ohrui, T.; Yamaya, M.; Arai, H. Effects of menthol on the triggering of the swallowing reflex in elderly patients with dysphagia. Br. J. Clin. Pharmacol. 2006, 62, 369–371. [Google Scholar] [CrossRef] [Green Version]
- Ebihara, T.; Ebihara, S.; Maruyama, M.; Kobayashi, M.; Itou, A.; Arai, H.; Sasaki, H. A randomized trial of olfactory stimulation using black pepper oil in older people with swallowing dysfunction. J. Am. Geriatr. Soc. 2006, 54, 1401–1406. [Google Scholar] [CrossRef]
- Munakata, M.; Kobayashi, K.; Niisato-Nezu, J.; Tanaka, S.; Kakisaka, Y.; Ebihara, T.; Ebihara, S.; Haginoya, K.; Tsuchiya, S.T.; Onuma, A. Olfactory stimulation using black pepper oil facilitates oral feeding in pediatric patients receiving long-term enteral nutrition. Tohoku J. Exp. Med. 2008, 214, 327–332. [Google Scholar] [CrossRef] [Green Version]
- Welge-Lüssen, A.; Ebnöther, M.; Wolfensberger, M.; Hummel, T. Swallowing is differentially influenced by retronasal compared with orthonasal stimulation in combination with gustatory stimuli. Chem. Senses 2009, 34, 499–502. [Google Scholar] [CrossRef] [Green Version]
- Pelletier, C.A.; Lawless, H.T. Effect of citric acid and citric acid-sucrose mixtures on swallowing in neurogenic oropharyngeal dysphagia. Dysphagia 2003, 18, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Roa Pauloski, B.; Logemann, J.A.; Rademaker, A.W.; Lundy, D.; Sullivan, P.A.; Newman, L.A.; Lazarus, C.; Bacon, M. Effects of enhanced bolus flavors on oropharyngeal swallow in patients treated for head and neck cancer. Head Neck 2013, 35, 1124–1131. [Google Scholar] [CrossRef] [PubMed]
- Mulheren, R.W.; Kamarunas, E.; Ludlow, C.L. Sour taste increases swallowing and prolongs hemodynamic responses in the cortical swallowing network. J. Neurophysiol. 2016, 116, 2033–2042. [Google Scholar] [CrossRef] [PubMed]
- Abdul Wahab, N.; Jones, R.D.; Huckabee, M.L. Effects of olfactory and gustatory stimuli on neural excitability for swallowing. Physiol. Behav. 2010, 101, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Miura, Y.; Morita, Y.; Koizumi, H.; Shingai, T. Effects of taste solutions, carbonation, and cold stimulus on the power frequency content of swallowing submental surface electromyography. Chem. Senses 2009, 34, 325–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pelletier, C.A.; Steele, C.M. Influence of the perceived taste intensity of chemesthetic stimuli on swallowing parameters given age and genetic taste differences in healthy adult women. J. SpeechLang. Hear. Res. 2014, 57, 46–56. [Google Scholar] [CrossRef] [Green Version]
- Hamdy, S.; Jilani, S.; Price, V.; Parker, C.; Hall, N.; Power, M. Modulation of human swallowing behaviour by thermal and chemical stimulation in health and after brain injury. Neurogastroenterol. Motil. 2003, 15, 69–77. [Google Scholar] [CrossRef]
- Nederkoorn, C.; Smulders, F.T.Y.; Jansen, A. Recording of swallowing events using electromyography as a non-invasive measurement of salivation. Appetite 1999, 33, 361–369. [Google Scholar] [CrossRef]
- Shingai, T.; Miyaoka, Y.; Ikarashi, R.; Shimada, K. Swallowing reflex elicited by water and taste solutions in humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1989, 256. [Google Scholar] [CrossRef]
- Pelletier, C.A.; Dhanaraj, G.E. The effect of taste and palatability on lingual swallowing pressure. Dysphagia 2006, 21, 121–128. [Google Scholar] [CrossRef]
- Chee, C.; Arshad, S.; Singh, S.; Mistry, S.; Hamdy, S. The influence of chemical gustatory stimuli and oral anaesthesia on healthy human pharyngeal swallowing. Chem. Senses 2005, 30, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Leow, L.P.; Huckabee, M.L.; Sharma, S.; Tooley, T.P. The influence of taste on swallowing apnea, oral preparation time, and duration and amplitude of submental muscle contraction. Chem. Senses 2007, 32, 119–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmer, P.M.; McCulloch, T.M.; Jaffe, D.; Neel, A.T. Effects of a sour bolus on the intramuscular electromyographic (EMG) activity of muscles in the submental region. Dysphagia 2005, 20, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Sciortino, K.F.; Liss, J.M.; Case, J.L.; Gerritsen, K.G.; Katz, R.C. Effects of mechanical, cold, gustatory, and combined stimulation to the human anterior faucial pillars. Dysphagia 2003, 18, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Ding, R.; Logemann, J.A.; Larson, C.R.; Rademaker, A.W. The effects of taste and consistency on swallow physiology in younger and older healthy individuals: A surface electromyographic study. J. Speech Lang. Hear. Res. 2003, 46, 977–989. [Google Scholar] [CrossRef]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature 1997, 389, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Lübbert, M.; Kyereme, J.; Schöbel, N.; Beltrán, L.; Wetzel, C.H.; Hatt, H. Transient Receptor Potential Channels Encode Volatile Chemicals Sensed by Rat Trigeminal Ganglion Neurons. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [Green Version]
- Szallasi, A.; Blumberg, P.M. Resiniferatoxin, a phorbol-related diterpene, acts as an ultrapotent analog of capsaicin, the irritant constituent in red pepper. Neuroscience 1989, 30, 515–520. [Google Scholar] [CrossRef] [Green Version]
- Premkumar, L.S. Transient receptor potential channels as targets for phytochemicals. ACS Chem. Neurosci. 2014, 5, 1117–1130. [Google Scholar] [CrossRef] [Green Version]
- Bisogno, T.; Hanuš, L.; De Petrocellis, L.; Tchilibon, S.; Ponde, D.E.; Brandi, I.; Moriello, A.S.; Davis, J.B.; Mechoulam, R.; Di Marzo, V. Molecular targets for cannabidiol and its synthetic analogues: Effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br. J. Pharmacol. 2001, 134, 845–852. [Google Scholar] [CrossRef]
- Yang, B.H.; Piao, Z.G.; Kim, Y.B.; Lee, C.H.; Lee, J.K.; Park, K.; Kim, J.S.; Oh, S.B. Activation of vanilloid receptor 1 (VR1) by Eugenol. J. Dent. Res. 2003, 82, 781–785. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Blair, N.T.; Clapham, D.E. Camphor activates and strongly desensitizes the transient receptor potential vanilloid subtype 1 channel in a vanilloid-independent mechanism. J. Neurosci. 2005, 25, 8924–8937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pearce, L.V.; Petukhov, P.A.; Szabo, T.; Kedei, N.; Bizik, F.; Kozikowski, A.P.; Blumberg, P.M. Evodiamine functions as an agonist for the vanilloid receptor TRPV1. Org. Biomol. Chem. 2004, 2, 2281–2286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morera, E.; De Petrocellis, L.; Morera, L.; Moriello, A.S.; Nalli, M.; Di Marzo, V.; Ortar, G. Synthesis and biological evaluation of [6]-gingerol analogues as transient receptor potential channel TRPV1 and TRPA1 modulators. Bioorganic Med. Chem. Lett. 2012, 22, 1674–1677. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, R.; Sato, T.; Yajima, T.; Kano, M.; Suzuki, T.; Ichikawa, H. The Distribution of TRPV1 and TRPV2 in the rat pharynx. Cell. Mol. Neurobiol. 2013, 33, 707–714. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Sato, Y.; Taniguchi, K. Distribution of TRPV1- and TRPV2-immunoreactive afferent nerve endings in rat trachea. J. Anat. 2007, 211, 775–783. [Google Scholar] [CrossRef]
- Kido, M.A.; Muroya, H.; Yamaza, T.; Terada, Y.; Tanaka, T. Vanilloid receptor expression in the rat tongue and palate. J. Dent. Res. 2003, 82, 393–397. [Google Scholar] [CrossRef]
- Hamamoto, T.; Takumida, M.; Hirakawa, K.; Takeno, S.; Tatsukawa, T. Localization of transient receptor potential channel vanilloid subfamilies in the mouse larynx. Acta Otolaryngol. 2008, 128, 685–693. [Google Scholar] [CrossRef]
- Wang, B.; Danjo, A.; Kajiya, H.; Okabe, K.; Kido, M.A. Oral epithelial cells are activated via TRP channels. J. Dent. Res. 2011, 90, 163–167. [Google Scholar] [CrossRef]
- Bán, Á.; Marincsák, R.; Bíró, T.; Perkecz, A.; Gömöri, É.; Sándor, K.; Tóth, I.B.; Bánvölgyi, Á.; Szolcsányi, J.; Pintér, E. Upregulation of transient receptor potential vanilloid type-1 receptor expression in oral lichen planus. Neuroimmunomodulation 2010, 17, 103–108. [Google Scholar] [CrossRef]
- Nakashimo, Y.; Takumida, M.; Fukuiri, T.; Anniko, M.; Hirakawa, K. Expression of transient receptor potential channel vanilloid (TRPV) 14, melastin (TRPM) 5 and 8, and ankyrin (TRPA1) in the normal and methimazole-treated mouse olfactory epithelium. Acta Otolaryngol. 2010, 130, 1278–1286. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.K.; Takumida, M.; Ishibashi, T.; Hamamoto, T.; Hirakawa, K. Expression of transient receptor potential vanilloid (TRPV) families 1, 2, 3 and 4 in the mouse olfactory epithelium. Rhinology 2009, 47, 242–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quartu, M.; Serra, M.P.; Boi, M.; Poddighe, L.; Picci, C.; Demontis, R.; Del Fiacco, M. TRPV1 receptor in the human trigeminal ganglion and spinal nucleus: Immunohistochemical localization and comparison with the neuropeptides CGRP and SP. J. Anat. 2016, 229, 755–767. [Google Scholar] [CrossRef] [PubMed]
- Zakir, H.M.; Mostafeezur, R.M.; Suzuki, A.; Hitomi, S.; Suzuki, I.; Maeda, T.; Seo, K.; Yamada, Y.; Yamamura, K.; Lev, S.; et al. Expression of TRPV1 Channels after Nerve Injury Provides an Essential Delivery Tool for Neuropathic Pain Attenuation. PLoS ONE 2012, 7, e44023. [Google Scholar] [CrossRef] [PubMed]
- Caterina, M.J.; Rosen, T.A.; Tominaga, M.; Brake, A.J.; Julius, D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature 1999, 398, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Berdugo, D.; Rofes, L.; Farré, R.; Casamitjana, J.F.; Enrique, A.; Chamizo, J.; Padrón, A.; Navarro, X.; Clavé, P. Localization and expression of TRPV1 and TRPA1 in the human oropharynx and larynx. Neurogastroenterol. Motil. 2016, 28, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Hamamoto, T.; Takumida, M.; Hirakawa, K.; Tatsukawa, T.; Ishibashi, T. Localization of transient receptor potential vanilloid (TRPV) in the human larynx. Acta Otolaryngol. 2009, 129, 560–568. [Google Scholar] [CrossRef]
- Seki, N.; Shirasaki, H.; Kikuchi, M.; Sakamoto, T.; Watanabe, N.; Himi, T. Expression and localization of TRPV1 in human nasal mucosa. Rhinology 2006, 44, 128–134. [Google Scholar]
- Cabib, C.; Nascimento, W.; Rofes, L.; Arreola, V.; Tomsen, N.; Mundet, L.; Palomeras, E.; Michou, E.; Clavé, P.; Ortega, O. Short-term neurophysiological effects of sensory pathway neurorehabilitation strategies on chronic poststroke oropharyngeal dysphagia. Neurogastroenterol. Motil. 2020. [Google Scholar] [CrossRef]
- Ebihara, S.; Maruyama, Y.; Ebihara, T.; Oshiro, T.; Kohzuki, M. Red wine polyphenols and swallowing reflex in dysphagia. Geriatr. Gerontol. Int. 2010, 10, 329–330. [Google Scholar] [CrossRef]
- Gonzalez, R.; Dunkel, R.; Koletzko, B.; Schusdziarra, V.; Allescher, H.D. Effect of capsaicin-containing red pepper sauce suspension on upper gastrointestinal motility in healthy volunteers. Dig. Dis. Sci. 1998, 43, 1165–1171. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; Vos, R.; Tack, J. Effects of capsaicin on the sensorimotor function of the proximal stomach in humans. Aliment. Pharmacol. Ther. 2004, 19, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Grossi, L.; Cappello, G.; Marzio, L. Effect of an acute intraluminal administration of capsaicin on oesophageal motor pattern in GORD patients with ineffective oesophageal motility. Neurogastroenterol. Motil. 2006, 18, 632–636. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.L.; Liu, T.T.; Yi, C.H.; Orr, W.C. Effects of capsaicin-containing red pepper sauce suspension on esophageal secondary peristalsis in humans. Neurogastroenterol. Motil. 2010, 22. [Google Scholar] [CrossRef] [PubMed]
- Story, G.M.; Peier, A.M.; Reeve, A.J.; Eid, S.R.; Mosbacher, J.; Hricik, T.R.; Earley, T.J.; Hergarden, A.C.; Andersson, D.A.; Hwang, S.W.; et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 2003, 112, 819–829. [Google Scholar] [CrossRef] [Green Version]
- Jaquemar, D.; Schenker, T.; Trueb, B. An ankyrin-like protein with transmembrane domains is specifically lost after oncogenic transformation of human fibroblasts. J. Biol. Chem. 1999, 274, 7325–7333. [Google Scholar] [CrossRef] [Green Version]
- Jordt, S.E.; Bautista, D.M.; Chuang, H.H.; McKemy, D.D.; Zygmunt, P.M.; Högestätt, E.D.; Meng, I.D.; Julius, D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 2004, 427, 260–265. [Google Scholar] [CrossRef]
- Bandell, M.; Story, G.M.; Hwang, S.W.; Viswanath, V.; Eid, S.R.; Petrus, M.J.; Earley, T.J.; Patapoutian, A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 2004, 41, 849–857. [Google Scholar] [CrossRef] [Green Version]
- Macpherson, L.J.; Geierstanger, B.H.; Viswanath, V.; Bandell, M.; Eid, S.R.; Hwang, S.W.; Patapoutian, A. The pungency of garlic: Activation of TRPA1 and TRPV1 in response to allicin. Curr. Biol. 2005, 15, 929–934. [Google Scholar] [CrossRef] [Green Version]
- Bautista, D.M.; Movahed, P.; Hinman, A.; Axelsson, H.E.; Sterner, O.; Högestätt, E.D.; Julius, D.; Jordt, S.E.; Zygmunt, P.M. Pungent products from garlic activate the sensory ion channel TRPA1. Proc. Natl. Acad. Sci. USA 2005, 102, 12248–12252. [Google Scholar] [CrossRef] [Green Version]
- Koizumi, K.; Iwasaki, Y.; Narukawa, M.; Iitsuka, Y.; Fukao, T.; Seki, T.; Ariga, T.; Watanabe, T. Diallyl sulfides in garlic activate both TRPA1 and TRPV1. Biochem. Biophys. Res. Commun. 2009, 382, 545–548. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.Q.; Ye, L.L.; Liu, X.L.; Qi, X.M.; Lv, J.D.; Wang, G.; Farhan, U.K.; Waqas, N.; Chen, D.D.; Han, L.; et al. Gingerol activates noxious cold ion channel TRPA1 in gastrointestinal tract. Chin. J. Nat. Med. 2016, 14, 434–440. [Google Scholar] [CrossRef]
- Xu, H.; Delling, M.; Jun, J.C.; Clapham, D.E. Oregano, thyme and clove-derived flavors and skin sensitizers activate specific TRP channels. Nat. Neurosci. 2006, 9, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Leamy, A.W.; Shukla, P.; McAlexander, M.A.; Carr, M.J.; Ghatta, S. Curcumin ((E,E)-1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione) activates and desensitizes the nociceptor ion channel TRPA1. Neurosci. Lett. 2011, 503, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Nassini, R.; Materazzi, S.; Vriens, J.; Prenen, J.; Benemei, S.; De Siena, G.; La Marca, G.; Andr, E.; Preti, D.; Avonto, C.; et al. The “headache tree” via umbellulone and TRPA1 activates the trigeminovascular system. Brain 2012, 135, 376–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, J.; Pollastro, F.; Prenen, J.; Zhu, Z.; Appendino, G.; Nilius, B. Ligustilide: A novel TRPA1 modulator. Pflug. Arch. Eur. J. Physiol. 2011, 462, 841–849. [Google Scholar] [CrossRef]
- Hu, H.; Bandell, M.; Petrus, M.J.; Zhu, M.X.; Patapoutian, A. Zinc activates damage-sensing TRPA1 ion channels. Nat. Chem. Biol. 2009, 5, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.; Ruei-Lung Lin, K. Heavy metals zinc, cadmium, and copper stimulate pulmonary sensory neurons via direct activation of TRPAl. J. Appl. Physiol. 2010, 108, 891–897. [Google Scholar] [CrossRef] [Green Version]
- McNamara, C.R.; Mandel-Brehm, J.; Bautista, D.M.; Siemens, J.; Deranian, K.L.; Zhao, M.; Hayward, N.J.; Chong, J.A.; Julius, D.; Moran, M.M.; et al. TRPA1 mediates formalin-induced pain. Proc. Natl. Acad. Sci. USA 2007, 104, 13525–13530. [Google Scholar] [CrossRef] [Green Version]
- Taylor-Clark, T.E.; Undem, B.J.; MacGlashan, D.W.; Ghatta, S.; Carr, M.J.; McAlexander, M.A. Prostaglandin-induced activation of nociceptive neurons via direct interaction with transient receptor potential A1 (TRPA1). Mol. Pharmacol. 2008, 73, 274–281. [Google Scholar] [CrossRef] [Green Version]
- Bessac, B.F.; Sivula, M.; Von Hehn, C.A.; Escalera, J.; Cohn, L.; Jordt, S.E. TRPA1 is a major oxidant sensor in murine airway sensory neurons. J. Clin. Investig. 2008, 118, 1899–1910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trevisani, M.; Siemens, J.; Materazzi, S.; Bautista, D.M.; Nassini, R.; Campi, B.; Imamachi, N.; Andrè, E.; Patacchini, R.; Cottrell, G.S.; et al. 4-Hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. Proc. Natl. Acad. Sci. USA 2007, 104, 13519–13524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersson, D.A.; Gentry, C.; Moss, S.; Bevan, S. Transient receptor potential A1 is a sensory receptor for multiple products of oxidative stress. J. Neurosci. 2008, 28, 2485–2494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richards, P.M.; Johnson, E.C.; Silver, W.L. Four irritating odorants target the trigeminal chemoreceptor TRPA1. Chemosens. Percept. 2010, 3, 190–199. [Google Scholar] [CrossRef]
- Martinez, J.M.; Eling, T.E. Activation of TRPA1 by volatile organic chemicals leading to sensory irritation. ALTEX 2019, 36, 572–582. [Google Scholar] [CrossRef]
- Wu, S.W.; Fowler, D.K.; Shaffer, F.J.; Lindberg, J.E.M.; Peters, J.H. Ethyl vanillin activates TRPA1. J. Pharmacol. Exp. Ther. 2017, 362, 368–377. [Google Scholar] [CrossRef] [Green Version]
- Kun, J.; Perkecz, A.; Knie, L.; Sétáló, G.; Tornóczki, T.; Pintér, E.; Bán, Á. TRPA1 receptor is upregulated in human oral lichen planus. Oral Dis. 2017, 23, 189–198. [Google Scholar] [CrossRef]
- Yu, S.; Gao, G.; Peterson, B.Z.; Ouyang, A. TRPA1 in mast cell activation-induced long-lasting mechanical hypersensitivity of vagal afferent C-fibers in guinea pig esophagus. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 297. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Hu, Y.; Yu, X.; Xi, J.; Fan, X.; Tse, C.M.; Myers, A.C.; Pasricha, P.J.; Li, X.; Yu, S. Allergen challenge sensitizes TRPA1 in vagal sensory neurons and afferent C-fiber subtypes in guinea pig esophagus. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 308, G482–G488. [Google Scholar] [CrossRef] [Green Version]
- Hondoh, A.; Ishida, Y.; Ugawa, S.; Ueda, T.; Shibata, Y.; Yamada, T.; Shikano, M.; Murakami, S.; Shimada, S. Distinct expression of cold receptors (TRPM8 and TRPA1) in the rat nodose-petrosal ganglion complex. Brain Res. 2010, 1319, 60–69. [Google Scholar] [CrossRef]
- Kim, Y.S.; Son, J.Y.; Kim, T.H.; Paik, S.K.; Dai, Y.; Noguchi, K.; Ahn, D.K.; Bae, Y.C. Expression of transient receptor potential ankyrin 1 (TRPA 1) in the rat trigeminal sensory afferents and spinal dorsal horn. J. Comp. Neurol. 2010, 518, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Nagata, K.; Duggan, A.; Kumar, G.; García-Añoveros, J. Nociceptor and hair cell transducer properties of TRPA1, a channel for pain and hearing. J. Neurosci. 2005, 25, 4052–4061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bharate, S.S.; Bharate, S.B. Modulation of thermoreceptor TRPM8 by cooling compounds. ACS Chem. Neurosci. 2012, 3, 248–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Qin, N. TRPM8 in health and disease: Cold sensing and beyond. Adv. Exp. Med. Biol. 2011, 704, 185–208. [Google Scholar]
- McKemy, D.D.; Neuhausser, W.M.; Julius, D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 2002, 416, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Peier, A.M.; Moqrich, A.; Hergarden, A.C.; Reeve, A.J.; Andersson, D.A.; Story, G.M.; Earley, T.J.; Dragoni, I.; McIntyre, P.; Bevan, S.; et al. A TRP channel that senses cold stimuli and menthol. Cell 2002, 108, 705–715. [Google Scholar] [CrossRef] [Green Version]
- Sato, T.; Fujita, M.; Kano, M.; Hosokawa, H.; Kondo, T.; Suzuki, T.; Kasahara, E.; Shoji, N.; Sasano, T.; Ichikawa, H. The distribution of transient receptor potential melastatin-8 in the rat soft palate, epiglottis, and pharynx. Cell. Mol. Neurobiol. 2013, 33, 161–165. [Google Scholar] [CrossRef]
- Zhang, L.; Jones, S.; Brody, K.; Costa, M.; Brookes, S.J.H. Thermosensitive transient receptor potential channels in vagal afferent neurons of the mouse. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 286. [Google Scholar] [CrossRef] [Green Version]
- Yajima, T.; Sato, T.; Hosokawa, H.; Kondo, T.; Saito, M.; Shimauchi, H.; Ichikawa, H. Distribution of transient receptor potential melastatin-8-containing nerve fibers in rat oral and craniofacial structures. Ann. Anat. 2015, 201, 1–5. [Google Scholar] [CrossRef]
- Abe, J.; Hosokawa, H.; Okazawa, M.; Kandachi, M.; Sawada, Y.; Yamanaka, K.; Matsumura, K.; Kobayashi, S. TRPM8 protein localization in trigeminal ganglion and taste papillae. Mol. Brain Res. 2005, 136, 91–98. [Google Scholar] [CrossRef]
- Viana, F. Chemosensory properties of the trigeminal system. ACS Chem. Neurosci. 2011, 2, 38–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damann, N.; Rothermel, M.; Klupp, B.G.; Mettenleiter, T.C.; Hatt, H.; Wetzel, C.H. Chemosensory properties of murine nasal and cutaneous trigeminal neurons identified by viral tracing. BMC Neurosci. 2006, 7. [Google Scholar] [CrossRef] [PubMed]
- Gerhold, K.A.; Bautista, D.M. Molecular and cellular mechanisms of trigeminal chemosensation. Annal. N. Y. Acad. Sci. 2009, 1170, 184–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roper, S.D. TRPs in taste and chemesthesis. Handb. Exp. Pharmacol. 2014, 223, 827–871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvarez-Berdugo, D.; Rofes, L.; Casamitjana, J.F.; Enrique, A.; Chamizo, J.; Viña, C.; Pollán, C.M.; Clavé, P. TRPM8, ASIC1, and ASIC3 localization and expression in the human oropharynx. Neurogastroenterol. Motil. 2018, 30. [Google Scholar] [CrossRef] [PubMed]
- Ebihara, T.; Ebihara, S.; Yamazaki, M.; Asada, M.; Yamanda, S.; Arai, H. Intensive stepwise method for oral intake using a combination of transient receptor potential stimulation and olfactory stimulation inhibits the incidence of pneumonia in dysphagic older adults. J. Am. Geriatr. Soc. 2010, 58, 196–198. [Google Scholar] [CrossRef]
- Kellenberger, S.; Schild, L. International union of basic and clinical pharmacology. XCI. structure, function, and pharmacology of acid-sensing ion channels and the epithelial Na+ channel. Pharmacol. Rev. 2015, 67, 1–35. [Google Scholar] [CrossRef] [Green Version]
- Li, W.G.; Xu, T. Le ASIC3 channels in multimodal sensory perception. ACS Chem. Neurosci. 2011, 2, 26–37. [Google Scholar] [CrossRef] [Green Version]
- Cristofori-Armstrong, B.; Rash, L.D. Acid-sensing ion channel (ASIC) structure and function: Insights from spider, snake and sea anemone venoms. Neuropharmacology 2017, 127, 173–184. [Google Scholar] [CrossRef] [Green Version]
- Fukuda, T.; Ichikawa, H.; Terayama, R.; Yamaai, T.; Kuboki, T.; Sugimoto, T. ASIC3-immunoreactive neurons in the rat vagal and glossopharyngeal sensory ganglia. Brain Res. 2006, 1081, 150–155. [Google Scholar] [CrossRef] [Green Version]
- Dusenkova, S.; Ru, F.; Surdenikova, L.; Nassenstein, C.; Hatok, J.; Dusenka, R.; Banovcin, P.; Kliment, J.; Kollarik, M.; Kollarik, M. The expression profile of acid-sensing ion channel (ASIC) subunits ASIC1a, ASIC1b, ASIC2a, ASIC2b, and ASIC3 in the esophageal vagal afferent nerve subtypes. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 307, G922–G930. [Google Scholar] [CrossRef] [PubMed]
- Rahman, F.; Harada, F.; Saito, I.; Suzuki, A.; Kawano, Y.; Izumi, K.; Nozawa-Inoue, K.; Maeda, T. Detection of acid-sensing ion channel 3 (ASIC3) in periodontal Ruffini endings of mouse incisors. Neurosci. Lett. 2011, 488, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Fang, P.; Hu, Z.; Ling, Y.; Liu, H. Mechanotransduction of trigeminal ganglion neurons innervating inner walls of rat anterior eye chambers. Am. J. Physiol. Cell Physiol. 2015, 309, C1–C10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ugawa, S.; Yamamoto, T.; Ueda, T.; Ishida, Y.; Inagaki, A.; Nishigaki, M.; Shimada, S. Amiloride-insensitive currents of the acid-sensing ion channel-2a (ASIC2a)/ASIC2b heteromeric sour-taste receptor channel. J. Neurosci. 2003, 23, 3616–3622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimada, S.; Ueda, T.; Ishida, Y.; Yamamoto, T.; Ugawa, S. Acid-sensing ion channels in taste buds. Arch. Histol. Cytol. 2006, 69, 227–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akiba, Y.; Mizumori, M.; Kuo, M.; Ham, M.; Guth, P.H.; Engel, E.; Kaunitz, J.D. CO2 chemosensing in rat oesophagus. Gut 2008, 57, 1654–1664. [Google Scholar] [CrossRef] [PubMed]
- Ru, F.; Banovcin, P.; Kollarik, M. Acid sensitivity of the spinal dorsal root ganglia C-fiber nociceptors innervating the guinea pig esophagus. Neurogastroenterol. Motil. 2015, 27, 865–874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- G.Khoo, S.; Al-Alawi, M.; Walsh, M.T.; Hannigan, K.; Glynn, S.; Thornton, M.; McQuaid, S.; Wang, Y.; Hamilton, P.W.; Verriere, V.; et al. Eosinophil peroxidase induces the expression and function of acid-sensing ion channel-3 in allergic rhinitis: In vitro evidence in cultured epithelial cells. Clin. Exp. Allergy 2012, 42, 1028–1039. [Google Scholar] [CrossRef] [PubMed]
- Nagy, A.; Steele, C.M.; Pelletier, C.A. Differences in swallowing between high and low concentration taste stimuli. Biomed. Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [Green Version]
- Humbert, I.A.; Joel, S. Tactile, gustatory, and visual biofeedback stimuli modulate neural substrates of deglutition. Dysphagia 2012, 27, 567. [Google Scholar] [CrossRef] [Green Version]
- Babaei, A.; Kern, M.; Antonik, S.; Mepani, R.; Ward, B.D.; Li, S.J.; Hyde, J.; Shaker, R. Enhancing effects of flavored nutritive stimuli on cortical swallowing network activity. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 299. [Google Scholar] [CrossRef]
- Elshukri, O.; Michou, E.; Mentz, H.; Hamdy, S. Brain and behavioral effects of swallowing carbonated water on the human pharyngeal motor system. J. Appl. Physiol. 2016, 120, 408–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinclair, W.J. Initiation of reflex swallowing from the naso- and oropharynx. Am. J. Physiol. 1970, 218, 956–960. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, W.J. Role of the pharyngeal plexus in initiation of swallowing. Am. J. Physiol. 1971, 221, 1260–1263. [Google Scholar] [CrossRef] [PubMed]
- Mostafeezur, R.M.; Zakir, H.M.; Takatsuji, H.; Yamada, Y.; Yamamura, K.; Kitagawa, J. Cannabinoids Facilitate the Swallowing Reflex Elicited by the Superior Laryngeal Nerve Stimulation in Rats. PLoS ONE 2012, 7, e50703. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, J.; Nakagawa, K.; Hasegawa, M.; Iwakami, T.; Shingai, T.; Yamada, Y.; Iwata, K. Facilitation of reflex swallowing from the pharynx and larynx. Dysphagia 2010, 25, 346. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, K.; Shingai, T.; Saito, I.; Yamamura, K.; Yamada, Y.; Kitagawa, J. Facilitation of the swallowing reflex with bilateral afferent input from the superior laryngeal nerve. Neurosci. Lett. 2014, 562, 50–53. [Google Scholar] [CrossRef]
- Guo, A. Immunocytochemical localization of the vanilloid receptor 1 (VR1): Relationship to neliropeptides, the P2X3 purinoceptor and IB4 binding sites. Eur. J. Neurosci. 1999, 11, 946–958. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, M.; Caterina, M.J.; Malmberg, A.B.; Rosen, T.A.; Gilbert, H.; Skinner, K.; Raumann, B.E.; Basbaum, A.I.; Julius, D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron 1998, 21, 531–543. [Google Scholar] [CrossRef] [Green Version]
- Mezey, É.; Tóth, Z.E.; Cortright, D.N.; Arzubi, M.K.; Krause, J.E.; Elde, R.; Guo, A.; Blumberg, P.M.; Szallasi, A. Distribution of mRNA for vanilloid receptor subtype 1 (VR1), and VR1-like immunoreactivity, in the central nervous system of the rat and human. Proc. Natl. Acad. Sci. USA 2000, 97, 3655–3660. [Google Scholar] [CrossRef]
- Peters, J.H.; McDougall, S.J.; Fawley, J.A.; Smith, S.M.; Andresen, M.C. Primary Afferent Activation of Thermosensitive TRPV1 Triggers Asynchronous Glutamate Release at Central Neurons. Neuron 2010, 65, 657–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peters, J.H.; McDougall, S.J.; Fawley, J.A.; Andresen, M.C. TRPV1 marks synaptic segregation of multiple convergent afferents at the rat medial solitary tract nucleus. PLoS ONE 2011, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shoudai, K.; Peters, J.H.; McDougall, S.J.; Fawley, J.A.; Andresen, M.C. Thermally active TRPV1 tonically drives central spontaneous glutamate release. J. Neurosci. 2010, 30, 14470–14475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doyle, M.W.; Bailey, T.W.; Jin, Y.H.; Andresen, M.C. Vanilloid receptors presynaptically modulate cranial visceral afferent synaptic transmission in nucleus tractus solitarius. J. Neurosci. 2002, 22, 8222–8229. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, M.E.; Andresen, M.C. Vanilloids selectively sensitize thermal glutamate release from TRPV1 expressing solitary tract afferents. Neuropharmacology 2016, 101, 401–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, Y.H.; Bailey, T.W.; Li, B.Y.; Schild, J.H.; Andresen, M.C. Purinergic and vanilloid receptor activation releases glutamate from separate cranial afferent terminals in nucleus tractus solitarius. J. Neurosci. 2004, 24, 4709–4717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bieger, D. Central nervous system control mechanisms of swallowing: A neuropharmacological perspective. Dysphagia 1993, 8, 308–310. [Google Scholar] [CrossRef] [PubMed]
- Ursu, D.; Knopp, K.; Beattie, R.E.; Liu, B.; Sher, E. Pungency of TRPV1 agonists is directly correlated with kinetics of receptor activation and lipophilicity. Eur. J. Pharmacol. 2010, 641, 114–122. [Google Scholar] [CrossRef]
- Liu, L.; Lo, Y.C.; Chen, I.J.; Simon, S.A. The responses of rat trigeminal ganglion neurons to capsaicin and two nonpungent vanilloid receptor agonists, olvanil and glyceryl nonamide. J. Neurosci. 1997, 17, 4101–4111. [Google Scholar] [CrossRef] [Green Version]
- Raisinghani, M.; Pabbidi, R.M.; Premkumar, L.S. Activation of transient receptor potential vanilloid 1 (TRPV1) by resiniferatoxin. J. Physiol. 2005, 567, 771–786. [Google Scholar] [CrossRef]
- Sörös, P.; Inamoto, Y.; Martin, R.E. Functional brain imaging of swallowing: An activation likelihood estimation meta-analysis. Hum. Brain Mapp. 2009, 30, 2426–2439. [Google Scholar] [CrossRef] [PubMed]
- Mihai, P.G.; Otto, M.; Platz, T.; Eickhoff, S.B.; Lotze, M. Sequential evolution of cortical activity and effective connectivity of swallowing using fMRI. Hum. Brain Mapp. 2014, 35, 5962–5973. [Google Scholar] [CrossRef]
- Hamdy, S.; Rothwell, J.C.; Brooks, D.J.; Bailey, D.; Aziz, Q.; Thompson, D.G. Identification of the cerebral loci processing human swallowing with H215O PET activation. J. Neurophysiol. 1999, 81, 1917–1926. [Google Scholar] [CrossRef] [Green Version]
- Paciaroni, M.; Mazzotta, G.; Corea, F.; Caso, V.; Venti, M.; Milia, P.; Silvestrelli, G.; Palmerini, F.; Parnetti, L.; Gallai, V. Dysphagia following stroke. Eur. Neurol. 2004, 51, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Fernandez, M.; Kleinman, J.T.; Ky, P.K.S.; Palmer, J.B.; Hillis, A.E. Supratentorial regions of acute ischemia associated with clinically important swallowing disorders: A pilot study. Stroke 2008, 39, 3022–3028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suntrup, S.; Kemmling, A.; Warnecke, T.; Hamacher, C.; Oelenberg, S.; Niederstadt, T.; Heindel, W.; Wiendl, H.; Dziewas, R. The impact of lesion location on dysphagia incidence, pattern and complications in acute stroke. Part 1: Dysphagia incidence, severity and aspiration. Eur. J. Neurol. 2015, 22, 832–838. [Google Scholar] [CrossRef]
- Hamdy, S.; Rothwell, J.C.; Aziz, Q.; Singh, K.D.; Thompson, D.G. Long-term reorganization of human motor cortex driven by short-term sensory stimulation. Nat. Neurosci. 1998, 1, 64–68. [Google Scholar] [CrossRef]
- Hamdy, S.; Aziz, Q.; Rothwell, J.C.; Hobson, A.; Barlow, J.; Thompson, D.G. Cranial nerve modulation of human cortical swallowing motor pathways. Am. J. Physiol. Gastrointest. Liver Physiol. 1997, 272. [Google Scholar] [CrossRef]
- Kline, D.D. Plasticity in glutamatergic NTS neurotransmission. Respir. Physiol. Neurobiol. 2008, 164, 105–111. [Google Scholar] [CrossRef] [Green Version]
- Bonham, A.C.; Chen, C.Y.; Sekizawa, S.I.; Joad, J.P. Plasticity in the nucleus tractus solitarius and its influence on lung and airway reflexes. J. Appl. Physiol. 2006, 101, 322–327. [Google Scholar] [CrossRef]
- Kline, D.D.; Ramirez-Navarro, A.; Kunze, D.L. Adaptive depression in synaptic transmission in the nucleus of the solitary tract after in vivo chronic intermittent hypoxia: Evidence for homeostatic plasticity. J. Neurosci. 2007, 27, 4663–4673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koizumi, S.; Fujishita, K.; Inoue, K.; Shigemoto-Mogami, Y.; Tsuda, M.; Inoue, K. Ca2+ waves in keratinocytes are transmitted to sensory neurons: The involvement of extracellular ATP and P2Y2 receptor activation. Biochem. J. 2004, 380, 329–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandadi, S.; Sokabe, T.; Shibasaki, K.; Katanosaka, K.; Mizuno, A.; Moqrich, A.; Patapoutian, A.; Fukumi-Tominaga, T.; Mizumura, K.; Tominaga, M. TRPV3 in keratinocytes transmits temperature information to sensory neurons via ATP. Pflug. Arch. Eur. J. Physiol. 2009, 458, 1093–1102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burrell, H.E.; Wlodarski, B.; Foster, B.J.; Buckley, K.A.; Sharpe, G.R.; Quayle, J.M.; Simpson, A.W.M.; Gallagher, J.A. Human keratinocytes release ATP and utilize three mechanisms for nucleotide interconversion at the cell surface. J. Biol. Chem. 2005, 280, 29667–29676. [Google Scholar] [CrossRef] [Green Version]
- Okada, S.F.; Nicholas, R.A.; Kreda, S.M.; Lazarowski, E.R.; Boucher, R.C. Physiological regulation of ATP release at the apical surface of human airway epithelia. J. Biol. Chem. 2006, 281, 22992–23002. [Google Scholar] [CrossRef] [Green Version]
- Tsai, T.L.; Chang, S.Y.; Ho, C.Y.; Yu, R.K. Role of ATP in the ROS-mediated laryngeal airway hyperreactivity induced by laryngeal acid-pepsin insult in anesthetized rats. J. Appl. Physiol. 2009, 106, 1584–1592. [Google Scholar] [CrossRef] [Green Version]
- Koizumi, H.; Ikezaki, S.; Ohbuchi, T.; Do, B.H.; Hohchi, N.; Kawaguchi, R.; Kitamura, T.; Suzuki, H. Acetylcholine-induced ex vivo ATP release from the human nasal mucosa. Auris Nasus Larynx 2017, 44, 422–427. [Google Scholar] [CrossRef]
- Ma, J.; Altomare, A.; Rieder, F.; Behar, J.; Biancani, P.; Harnett, K.M. ATP: A mediator for HCL-induced TRPV1 activation in esophageal mucosa. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 301. [Google Scholar] [CrossRef]
- Wu, L.; Oshima, T.; Shan, J.; Sei, H.; Tomita, T.; Ohda, Y.; Fukui, H.; Watari, J.; Miwa, H. PAR-2 activation enhances weak acid-induced ATP release through TRPV1 and ASIC sensitization in human esophageal epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, G695–G702. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Oshima, T.; Fukui, H.; Watari, J.; Miwa, H. Adenosine triphosphate induces P2Y2 activation and interleukin-8 release in human esophageal epithelial cells. J. Gastroenterol. Hepatol. 2017, 32, 1341–1347. [Google Scholar] [CrossRef]
- Takahashi, N.; Nakamuta, N.; Yamamoto, Y. Morphology of P2X3-immunoreactive nerve endings in the rat laryngeal mucosa. Histochem. Cell Biol. 2016, 145, 131–146. [Google Scholar] [CrossRef] [PubMed]
- Soda, Y.; Yamamoto, Y. Morphology and chemical characteristics of subepithelial laminar nerve endings in the rat epiglottic mucosa. Histochem. Cell Biol. 2012, 138, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Prasad, M.; Fearon, I.M.; Zhang, M.; Laing, M.; Vollmer, C.; Nurse, C.A. Expression of P2X2 and P2X3 receptor subunits in rat carotid body afferent neurones: Role in chemosensory signalling. J. Physiol. 2001, 537, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Feng, D.; Yan, H.; Wang, Z.; Pei, L. Comparative analysis of P2X1, P2X2, P2X3, and P2X4 receptor subunits in rat nodose ganglion neurons. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruan, H.Z.; Burnstock, G. Localisation of P2Y1 and P2Y4 receptors in dorsal root, nodose and trigeminal ganglia of the rat. Histochem. Cell Biol. 2003, 120, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Hubscher, C.H.; Petruska, J.C.; Rau, K.K.; Johnson, R.D. Co-expression of P2X receptor subunits on rat nodose neurons that bind the isolectin GS-I-B4. Neuroreport 2001, 12, 2995–2997. [Google Scholar] [CrossRef] [PubMed]
- Staikopoulos, V.; Sessle, B.J.; Furness, J.B.; Jennings, E.A. Localization of P2X2 and P2X3 receptors in rat trigeminal ganglion neurons. Neuroscience 2007, 144, 208–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suntrup-Krueger, S.; Bittner, S.; Recker, S.; Meuth, S.G.; Warnecke, T.; Suttrup, I.; Marian, T.; Dziewas, R. Electrical pharyngeal stimulation increases substance P level in saliva. Neurogastroenterol. Motil. 2016, 28, 855–860. [Google Scholar] [CrossRef]
- Muhle, P.; Suntrup-Krueger, S.; Bittner, S.; Ruck, T.; Claus, I.; Marian, T.; Schröder, J.B.; Minnerup, J.; Warnecke, T.; Meuth, S.G.; et al. Increase of substance P concentration in saliva after pharyngeal electrical stimulation in severely dysphagic stroke patients -An indicator of decannulation success? NeuroSignals 2018, 25, 74–87. [Google Scholar] [CrossRef]
- Caldeira, D.; Alarcão, J.; Vaz-Carneiro, A.; Costa, J. Risk of pneumonia associated with use of angiotensin converting enzyme inhibitors and angiotensin receptor blockers: Systematic review and meta-analysis. BMJ 2012, 345. [Google Scholar] [CrossRef] [Green Version]
- Arai, T.; Yasuda, Y.; Toshima, S.; Yoshimi, N.; Kashiki, Y. ACE inhibitors and pneumonia in elderly people [6]. Lancet 1998, 352, 1937–1938. [Google Scholar] [CrossRef]
- Nakayama, K.; Sekizawa, K.; Sasaki, H. ACE inhibitor and swallowing reflex [3]. Chest 1998, 113, 1425. [Google Scholar] [CrossRef] [PubMed]
- Ohkubo, T.; Chapman, N.; Neal, B.; Woodward, M.; Omae, T.; Chalmers, J. Effects of an angiotensin-converting enzyme inhibitor-based regimen on pneumonia risk. Am. J. Respir. Crit. Care Med. 2004, 169, 1041–1045. [Google Scholar] [CrossRef] [PubMed]
- Miarons, M.; Tomsen, N.; Nascimento, W.; Espín; López-Faixó, D.; Clavé, P.; Rofes, L. Increased levels of substance P in patients taking beta-blockers are linked with a protective effect on oropharyngeal dysphagia. Neurogastroenterol. Motil. 2018, 30. [Google Scholar] [CrossRef] [PubMed]
- Terenghi, G.; Polak, J.M.; Rodrigo, J.; Mulderry, P.K.; Bloom, S.R. Calcitonin gene-related peptide-immunoreactive nerves in the tongue, epiglottis and pharynx of the rat: Occurrence, distribution and origin. Brain Res. 1986, 365, 1–14. [Google Scholar] [CrossRef]
- Baluk, P.; Nadel, J.A.; McDonald, D.M. Substance P-immunoreactive sensory axons in the rat respiratory tract: A quantitative study of their distribution and role in neurogenic inflammation. J. Comp. Neurol. 1992, 319, 586–598. [Google Scholar] [CrossRef]
- Smithard, D.G. Substance P and swallowing after stroke. Therapy 2006, 3, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, T.; Kuwahara-Otani, S.; Maeda, S.; Tanaka, K.; Seki, M. Calcitonin gene-related peptide immunoreactive sensory neurons in the vagal and glossopharyngeal ganglia innervating the larynx of the rat. J. Chem. Neuroanat. 2014, 55, 18–23. [Google Scholar] [CrossRef]
- Domeij, S.; Dahlqvist, Å.; Forsgren, S. Regional differences in the distribution of nerve fibers showing substance P- and calcitonin gene-related peptide-like immunoreactivity in the rat larynx. Anat. Embryol. 1991, 183, 49–56. [Google Scholar] [CrossRef]
- Simons, C.T.; Klein, A.H.; Carstens, E. Chemogenic Subqualities of Mouthfeel. Chem. Senses 2019, 44, 281–288. [Google Scholar] [CrossRef]
- Marfurt, C.F.; Rajchert, D.M. Trigeminal primary afferent projections to “non-trigeminal” areas of the rat central nervous system. J. Comp. Neurol. 1991, 303, 489–511. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, K.; Arvidsson, J. Central distribution of trigeminal and upper cervical primary afferents in the rat studied by anterograde transport of horseradish peroxidase conjugated to wheat germ agglutinin. J. Comp. Neurol. 1988, 268, 91–108. [Google Scholar] [CrossRef] [PubMed]
- Saito, H.; Katagiri, A.; Okada, S.; Mikuzuki, L.; Kubo, A.; Suzuki, T.; Ohara, K.; Lee, J.; Gionhaku, N.; Iinuma, T.; et al. Ascending projections of nociceptive neurons from trigeminal subnucleus caudalis: A population approach. Exp. Neurol. 2017, 293, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Tsujimura, T.; Kondo, M.; Kitagawa, J.; Tsuboi, Y.; Saito, K.; Tohara, H.; Ueda, K.; Sessle, B.J.; Iwata, K. Involvement of ERK phosphorylation in brainstem neurons in modulation of swallowing reflex in rats. J. Physiol. 2009, 587, 805–817. [Google Scholar] [CrossRef] [PubMed]
- Bieger, D.; Neuhuber, W. Neural circuits and mediators regulating swallowing in the brainstem. GI Motil. Online 2006, 1–19. [Google Scholar] [CrossRef]
- Patrickson, J.W.; Smith, T.E.; Zhou, S.S. Afferent projections of the superior and recurrent laryngeal nerves. Brain Res. 1991, 539, 169–174. [Google Scholar] [CrossRef]
- Wank, M.; Neuhuber, W.L. Local differences in vagal afferent innervation of the rat esophagus are reflected by neurochemical differences at the level of the sensory ganglia and by different brainstem projections. J. Comp. Neurol. 2001, 435, 41–59. [Google Scholar] [CrossRef]
- Hayakawa, T.; Takanaga, A.; Maeda, S.; Seki, M.; Yajima, Y. Subnuclear distribution of afferents from the oral, pharyngeal and laryngeal regions in the nucleus tractus solitarii of the rat: A study using transganglionic transport of cholera toxin. Neurosci. Res. 2001, 39, 221–232. [Google Scholar] [CrossRef]
- Altschuler, S.M.; Bao, X.; Bieger, D.; Hopkins, D.A.; Miselis, R.R. Viscerotopic representation of the upper alimentary tract in the rat: Sensory ganglia and nuclei of the solitary and spinal trigeminal tracts. J. Comp. Neurol. 1989, 283, 248–268. [Google Scholar] [CrossRef]
- Iannilli, E.; Gudziol, V. Gustatory pathway in humans: A review of models of taste perception and their potential lateralization. J. Neurosci. Res. 2019, 97, 230–240. [Google Scholar] [CrossRef]
- Simon, S.A.; De Araujo, I.E.; Gutierrez, R.; Nicolelis, M.A.L. The neural mechanisms of gustation: A distributed processing code. Nat. Rev. Neurosci. 2006, 7, 890–901. [Google Scholar] [CrossRef] [PubMed]
- Roper, S.D.; Chaudhari, N. Taste buds: Cells, signals and synapses. Nat. Rev. Neurosci. 2017, 18, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Weiss, M.S.; Hajnal, A.; Czaja, K.; Di Lorenzo, P.M. Taste Responses in the Nucleus of the Solitary Tract of Awake Obese Rats Are Blunted Compared With Those in Lean Rats. Front. Integr. Neurosci. 2019, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradley, R.M. Sensory receptors of the larynx. Am. J. Med. 2000, 108, 47–50. [Google Scholar] [CrossRef]
- Arai, T.; Ohkuri, T.; Yasumatsu, K.; Kaga, T.; Ninomiya, Y. The role of transient receptor potential vanilloid-1 on neural responses to acids by the chorda tympani, glossopharyngeal and superior laryngeal nerves in mice. Neuroscience 2010, 165, 1476–1489. [Google Scholar] [CrossRef] [PubMed]
- Gilbertson, T.A.; Avenet, P.; Kinnamon, S.C.; Roper, S.D. Proton currents through amiloride-sensitive na channels in hamster taste cells: Role in acid transduction. J. Gen. Physiol. 1992, 100, 803–824. [Google Scholar] [CrossRef] [Green Version]
- Stevens, D.R.; Seifert, R.; Bufe, B.; Müller, F.; Kremmer, E.; Gauss, R.; Meyerhof, W.; Kaupp, U.B.; Lindemann, B. Hyperpolarization-activated channels HCN1 and HCN4 mediate responses to sour stimuli. Nature 2001, 413, 631–635. [Google Scholar] [CrossRef] [PubMed]
- Ishimaru, Y.; Inada, H.; Kubota, M.; Zhuang, H.; Tominaga, M.; Matsunami, H. Transient receptor potential family members PKD1L3 and PKD2L1 form a candidate sour taste receptor. Proc. Natl. Acad. Sci. USA 2006, 103, 12569–12574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, W.; Chang, R.B.; Bushman, J.D.; Tu, Y.H.; Mulhall, E.M.; Wilson, C.E.; Cooper, A.J.; Chick, W.S.; Hill-Eubanks, D.C.; Nelson, M.T.; et al. The K+ channel KIR2.1 functions in tandem with proton influx to mediate sour taste transduction. Proc. Natl. Acad. Sci. USA 2016, 113, E229–E238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teng, B.; Wilson, C.E.; Tu, Y.H.; Joshi, N.R.; Kinnamon, S.C.; Liman, E.R. Cellular and Neural Responses to Sour Stimuli Require the Proton Channel Otop1. Curr. Biol. 2019, 29, 3647–3656.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michou, E.; Mastan, A.; Ahmed, S.; Mistry, S.; Hamdy, S. Examining the role of carbonation and temperature on water swallowing performance: A swallowing reaction-time study. Chem. Senses 2012, 37, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Bülow, M.; Olsson, R.; Ekberg, O. Videoradiographic Analysis of How Carbonated Thin Liquids and Thickened Liquids Affect the Physiology of Swallowing in Subjects with Aspiration on Thin Liquids. Acta Radiol. 2003, 44, 366–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moritaka, H.; Kitade, M.; Sawamura, S.-i.; Takihara, T.; Awano, I.; Ono, T.; Tamine, K.; Hori, K. Effect of carbon dioxide in carbonated drinks on linguapalatal swallowing pressure. Chem. Senses 2014, 39, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Morishita, M.; Mori, S.; Yamagami, S.; Mizutani, M. Effect of carbonated beverages on pharyngeal swallowing in young individuals and elderly inpatients. Dysphagia 2014, 29, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Larsson, V.; Torisson, G.; Bülow, M.; Londos, E. Effects of carbonated liquid on swallowing dysfunction in dementia with Lewy bodies and Parkinson’s disease dementia. Clin. Interv. Aging 2017, 12, 1215–1222. [Google Scholar] [CrossRef] [Green Version]
- Sdravou, K.; Walshe, M.; Dagdilelis, L. Effects of carbonated liquids on oropharyngeal swallowing measures in people with neurogenic dysphagia. Dysphagia 2012, 27, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Hewson, L.; Hollowood, T.; Chandra, S.; Hort, J. Gustatory, olfactory and trigeminal interactions in a model carbonated beverage. Chemosens. Percept. 2009, 2, 94–107. [Google Scholar] [CrossRef]
- Yau, N.J.N.; Mcdaniel, M.R. The Power Function of Carbonation. J. Sens. Stud. 1990, 5, 117–128. [Google Scholar] [CrossRef]
- Wise, P.M.; Bryant, B. The effect of temperature and menthol on carbonation bite. Chem. Senses 2014, 39. [Google Scholar] [CrossRef]
- Dessirier, J.M.; Simons, C.T.; Carstens, M.I.; O’Mahony, M.; Carstens, E. Psychophysical and neurobiological evidence that the oral sensation elicited by carbonated water is of chemogenic origin. Chem. Senses 2000, 25, 277–284. [Google Scholar] [CrossRef] [Green Version]
- Simons, C.T.; Dessirier, J.-M.; Iodi Carstens, M.; O’Mahony, M.; Carstens, E. Erratum: Neurobiological and psychophysical mechanisms underlying the oral sensation produced by carbonated water. J. Neurosci. 1999, 19, 10191. [Google Scholar]
- Komai, M.; Bryant, B.P. Acetazolamide specifically inhibits lingual trigeminal nerve responses to carbon dioxide. Brain Res. 1993, 612, 122–129. [Google Scholar] [CrossRef]
- Supuran, C. Carbonic Anhydrases as Drug Targets—An Overview. Curr. Top. Med. Chem. 2007, 7, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Belmonte, C.; Rang, H.P. Capsaicin and carbon dioxide act by distinct mechanisms on sensory nerve terminals in the cat cornea. Pain 1997, 70, 23–29. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Chang, R.B.; Liman, E.R. TRPA1 is a component of the nociceptive response to CO2. J. Neurosci. 2010, 30, 12958–12963. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekar, J.; Yarmolinsky, D.; Von Buchholtz, L.; Oka, Y.; Sly, W.; Ryba, N.J.P.; Zuker, C.S. The taste of carbonation. Science 2009, 326, 443–445. [Google Scholar] [CrossRef] [Green Version]
- Lossow, K.; Hermans-Borgmeyer, I.; Behrens, M.; Meyerhof, W. Genetic labeling of Car4-expressing cells reveals subpopulations of type III taste cells. Chem. Senses 2017, 42, 747–758. [Google Scholar] [CrossRef]
- Ziemann, A.E.; Allen, J.E.; Dahdaleh, N.S.; Drebot, I.I.; Coryell, M.W.; Wunsch, A.M.; Lynch, C.M.; Faraci, F.M.; Howard, M.A.; Welsh, M.J.; et al. The Amygdala Is a Chemosensor that Detects Carbon Dioxide and Acidosis to Elicit Fear Behavior. Cell 2009, 139, 1012–1021. [Google Scholar] [CrossRef] [Green Version]
- Trapp, S.; Aller, M.I.; Wisden, W.; Gourine, A. V A role for TASK-1 (KCNK3) channels in the chemosensory control of breathing. J. Neurosci. 2008, 28, 8844–8850. [Google Scholar] [CrossRef] [Green Version]
- Masilamoney, M.; Dowse, R. Knowledge and practice of healthcare professionals relating to oral medicine use in swallowing-impaired patients: A scoping review. Int. J. Pharm. Pract. 2018, 26, 199–209. [Google Scholar] [CrossRef] [Green Version]
- Sato, D.; Sato, T.; Urata, Y.; Okajima, T.; Kawamura, S.; Kurita, M.; Takahashi, K.; Nanno, M.; Watahiki, A.; Kokubun, S.; et al. Distribution of TRPVs, P2X3, and parvalbumin in the human nodose ganglion. Cell. Mol. Neurobiol. 2014, 34, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Yajima, T.; Sato, T.; Shimazaki, K.; Ichikawa, H. Transient receptor potential melastatin-3 in the rat sensory ganglia of the trigeminal, glossopharyngeal and vagus nerves. J. Chem. Neuroanat. 2019, 96, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Sprunger, L.K.; Simasko, S.M. Expression of transient receptor potential channels and two-pore potassium channels in subtypes of vagal afferent neurons in rat. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 298, G212. [Google Scholar] [CrossRef] [PubMed] [Green Version]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hossain, M.Z.; Ando, H.; Unno, S.; Kitagawa, J. Targeting Chemosensory Ion Channels in Peripheral Swallowing-Related Regions for the Management of Oropharyngeal Dysphagia. Int. J. Mol. Sci. 2020, 21, 6214. https://doi.org/10.3390/ijms21176214
Hossain MZ, Ando H, Unno S, Kitagawa J. Targeting Chemosensory Ion Channels in Peripheral Swallowing-Related Regions for the Management of Oropharyngeal Dysphagia. International Journal of Molecular Sciences. 2020; 21(17):6214. https://doi.org/10.3390/ijms21176214
Chicago/Turabian StyleHossain, Mohammad Zakir, Hiroshi Ando, Shumpei Unno, and Junichi Kitagawa. 2020. "Targeting Chemosensory Ion Channels in Peripheral Swallowing-Related Regions for the Management of Oropharyngeal Dysphagia" International Journal of Molecular Sciences 21, no. 17: 6214. https://doi.org/10.3390/ijms21176214
APA StyleHossain, M. Z., Ando, H., Unno, S., & Kitagawa, J. (2020). Targeting Chemosensory Ion Channels in Peripheral Swallowing-Related Regions for the Management of Oropharyngeal Dysphagia. International Journal of Molecular Sciences, 21(17), 6214. https://doi.org/10.3390/ijms21176214