Glutathione and Glutathione-Like Sequences of Opioid and Aminergic Receptors Bind Ascorbic Acid, Adrenergic and Opioid Drugs Mediating Antioxidant Function: Relevance for Anesthesia and Abuse
Abstract
:1. Introduction
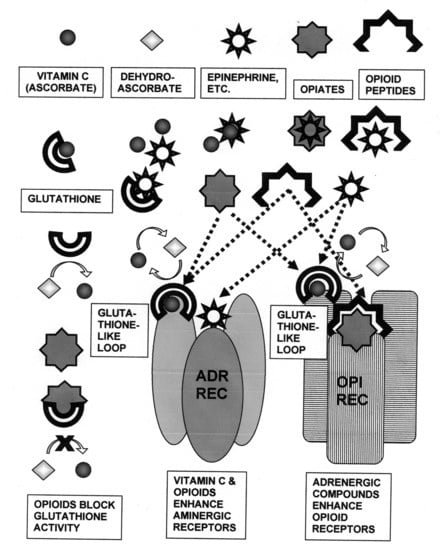
2. Results
3. Discussion
4. Materials and Methods
4.1. Ligands
4.2. Opioid Receptor Peptide Synthesis and Preparation
4.3. Peptide Binding Test Methods
4.4. Data Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Meister, A.; Tate, S.S. Glutathione and related γ-glutamyl compounds; biosynthesis and utilization. Ann. Rev. Biochem. 1976, 45, 550–604. [Google Scholar] [CrossRef] [PubMed]
- Pizzorno, J. Glutathione! Integr. Med. (Encinitas) 2014, 13, 8–12. [Google Scholar] [PubMed]
- Skoulis, N.P.; James, R.C.; Harbison, R.D.; Roberts, S.M. Depression of hepatic glutathione by opioid analgesic drugs in mice. Toxicol. Appl. Pharmacol. 1989, 99, 139–147. [Google Scholar] [CrossRef]
- Cemek, M.; Büyükokuroğlu, M.E.; Hazman, Ö.; Bulut, S.; Konuk, M.; Birdane, Y. Antioxidant enzyme and element status in heroin addiction or heroin withdrawal in rats: Effect of melatonin and vitamin E plus Se. Biol. Trace Elem. Res. 2011, 139, s41–s54. [Google Scholar] [CrossRef]
- Skrabalova, J.; Drastichova, Z.; Novotny, J. Morphine as a potential oxidative stress-causing agent. Mini Rev. Org. Chem. 2013, 10, 367–372. [Google Scholar] [CrossRef] [Green Version]
- Zahmatkesh, M.; Kadkhodaee, M.; Salarian, A.; Seifi, B.; Adel, I.S. Impact of opioids on oxidative status and related signaling pathways: An integrated view. J. Opioid. Manag. 2017, 13, 241–251. [Google Scholar] [CrossRef]
- Salarian, A.; Kadkhodaee, M.; Zahmatkesh, M.; Seifi, B.; Bakhshi, E.; Akhondzadeh, S.; Adeli, S.; Askari, H.; Arbabi, M. Opioid use disorder induces oxidative stress and inflammation; the attenuating effect of methadone maintenance treatment. Iran. J. Psychiatry 2018, 13, 46–54. [Google Scholar]
- Lastbom, L.E.; Moldéus, P.; Orrenius, S. On the mechanisms of glutathione depletion in hepatocytes exposed to morphine and ethylmorphine. Toxicology 1986, 42, 13–21. [Google Scholar] [CrossRef]
- Gómez-Lechón, M.J.; Ponsoda, X.; Jover, R.; Fabra, R.; Trullenque, R.; Castell, J.V. Hepatotoxicity of the opioids morphine, heroin, meperidine, and methadone to cultured human hepatocytes. Mol. Toxicol. 1987, 1, 453–463. [Google Scholar]
- Todaka, T.; Ishida, T.; Kita, H.; Narimatsu, S.; Yamano, S. Bioactivation of morphine in human liver; isolation and identification of morphinone, a toxic metabolite. Biol. Pharm. Bull. 2005, 28, 1275–1280. [Google Scholar] [CrossRef] [Green Version]
- Mannelli, P.; Patkar, A.; Rozen, S.; Matson, W.; Krishnan, R.; Kaddurah-Daouk, R. Opioid use affects antioxidant activity and purine metabolism: Preliminary results. Hum. Psychopharmacol. 2009, 24, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Leventelis, C.; Goutzourelas, N.; Kortsinidou, A.; Spanidis, Y.; Toulia, G.; Kampitsi, A.; Tsitsimpikou, C.; Stagos, D.; Veskoukis, A.S.; Kouretas, D. Buprenorphine and methadone as opioid maintenance treatments for heroin-addicted patients induce oxidative stress in blood. Oxid. Med. Cell Longev. 2019, 2019, 9417048. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Zhang, Q.; Zhang, Y.; Ouyang, Z.; Zheng, Q.; Zheng, R. Oxidative stress in heroin administered mice and natural antioxidants protection. Life Sci. 2005, 77, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Wang, Z.; Li, G.; Li, B.; Lin, H.; Zheng, R.; Zheng, Q. Heroin-administered mice involved in oxidative stress and exogenous antioxidant-alleviated withdrawal syndrome. Basic Clin. Pharmacol. Toxicol. 2006, 99, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, D.C.; Vázquez, I.E.; Brizuela, N.O.; Alvarez, R.G.; Mejía, G.B.; García, E.H.; Santamaría, D.; de Apreza, M.L.; Olguín, H.J. Assessment of oxidative damage induced by acute doses of morphine sulfate in postnatal and adult rat brain. Neurochem. Res. 2006, 31, 549–554. [Google Scholar] [CrossRef]
- Guzmán, D.C.; Brizuela, N.O.; Alvarez, R.G.; García, E.H.; Mejía, G.B.; Olguín, H.J. Cerebrolysin and morphine decrease glutathione and 5-hydroxyindole acetic acid levels in fasted rat brain. Biomed. Pharmacother. 2009, 63, 517–621. [Google Scholar] [CrossRef]
- Gutowicz, M.; Sadurska, B.; Chołojczyk, M.; Pokorska-Lis, M.; Siwińska-Ziółkowska, A.; Barańczyk-Kuźma, A. Antioxidant status in different regions of heroin addicts’ brain. Environ. Toxicol. Pharmacol. 2006, 21, 80–85. [Google Scholar] [CrossRef]
- Gutowicz, M.; Kaźmierczak, B.; Barańczyk-Kuźma, A. The influence of heroin abuse on glutathione-dependent enzymes in human brain. Drug Alcohol. Depend. 2011, 113, 8–12. [Google Scholar] [CrossRef]
- Tong, J.; Fitzmaurice, P.S.; Moszczynska, A.; Rathitharan, G.; Ang, L.C.; Meyer, J.H.; Mizrahi, R.; Boileau, I.; Furukawa, Y.; McCluskey, T.; et al. Normal glutathione levels in autopsied brain of chronic users of heroin and of cocaine. Drug Alcohol. Depend. 2018, 190, 20–28. [Google Scholar] [CrossRef]
- Holmquist, G.L. Opioid metabolism and effects of cytochrome P450. Pain Med. 2009, 10, S20–S29. [Google Scholar] [CrossRef] [Green Version]
- Projean, D.; Morin, P.-E.; Tu, T.M.; Ducharme, J. Identification of CYP3A4 and CYP2C8 as the major cytochrome P450 s responsible for morphine N –demethylation in human liver microsomes. Xenobiotica 2003, 33, 841–854. [Google Scholar] [CrossRef] [PubMed]
- Amunugama, H.T.; Zhang, H.; Hollenberg, P.F. Mechanism-based inactivation of cytochrome P450 2B6 by methadone through destruction of prosthetic heme. Drug Metab. Dispos. 2012, 40, 1765–1770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Amunugama, H.; Ney, S.; Cooper, N.; Hollenberg, P.F. Mechanism-based inactivation of human cytochrome P450 2B6 by clopidogrel; involvement of both covalent modification of cysteinyl residue 475 and loss of heme. Mol. Pharmacol. 2011, 80, 839–847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myers, A.L.; Hassan, H.E.; Lee, I.J.; Eddington, N.D. Repeated administration of oxycodone modifies the gene expression of several drug metabolising enzymes in the hepatic tissue of male Sprague-Dawley rats.; including glutathione S-transferase A-5 (rGSTA5) and CYP3A2. J. Pharm. Pharmacol. 2010, 62, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Yamano, S.; Takahashi, A.; Todaka, T.; Toki, S. In vivo and in vitro formation of morphinone from morphine in rat. Xenobiotica 1997, 27, 645–656. [Google Scholar] [CrossRef] [PubMed]
- Ishida, T.; Yamano, S.; Toki, S. Detection of morphinone as a new metabolite of morphine in guinea pig urine. Advances in Endogenous and Exogenous Opioids. In Proceedings of the International Narcotic Research Conference (Satellite Symposium of the 8th International Congress of Pharmacology), Kyoto, Japan, 26–30 July 1981; pp. 481–483. [Google Scholar] [CrossRef]
- Misra, A.L.; Yeh, S.H.; Woods, L.A. Morphine conjugates in the dog. Biochem. Pharmacol. 1970, 19, 1536–1539. [Google Scholar] [CrossRef]
- Nagamatsu, K.; Kido, Y.; Terao, T.; Ishida, T.; Toki, S. Protective effect of sulfhydryl compounds on acute toxicity of morphinone. Life Sci. 1982, 30, 1121–1127. [Google Scholar] [CrossRef]
- Garadnay, S.; Gyulai, Z.; Makleit, S.; Sipos, A. First synthesis of important secondary oxidative metabolites of morphine and codeine with the Michael addition. Cent. Eur. J. Chem. 2013, 11, 430–437. [Google Scholar] [CrossRef] [Green Version]
- Schneider, K.J. Covalent Protein Adduction by Drugs of Abuse. Ph.D. Thesis, Florida International University, Miami, FL, USA, 27 February 2013. [Google Scholar]
- Nagamatsu, K.; Hasegawa, A. Covalent binding of morphine to isolated rat hepatocytes. Biochem. Pharmacol. 1992, 43, 2631–2635. [Google Scholar]
- Ponsoda, X.; Jover, R.; Gómez-Lechón, M.J.; Fabra, R.; Trullenque, R.; Castell, J.V. Intracellular glutathione in human hepatocytes incubated with S-adenosyl-L-methionine and GSH-depleting drugs. Toxicology 1991, 70, 293–302. [Google Scholar] [CrossRef]
- Yun, J.; Oliynyk, S.; Lee, Y.; Kim, J.; Yun, K.; Jeon, R.; Ryu, J.H.; Oh, S. Ajoene restored behavioral patterns and liver glutathione level in morphine treated C57BL6 mice. Arch. Pharm. Res. 2017, 40, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Correia, M.A.; Krowech, G.; Caldera-Munoz, P.; Yee, S.L.; Straub, K.; Castagnoli, N., Jr. Morphine metabolism revisited. II. Isolation and chemical characterization of a glutathionylmorphine adduct from rat liver microsomal preparations. Chem. Biol. Interact. 1984, 51, 13–24. [Google Scholar] [CrossRef]
- Ishida, T.; Kumagai, Y.; Ikeda, Y.; Ito, K.; Yano, M.; Tok, I.S.; Mihashi, K.; Fujioka, T.; Iwase, Y.; Hachiyama, S. (8S)-(glutathion-S-yl)dihydromorphinone: Novel metabolite of morphine from guinea pig bile. Drug Metab. Dispos. 1989, 17, 77–81. [Google Scholar] [PubMed]
- Kumagai, Y.; Todaka, T.; Toki, S. A new metabolic pathway of morphine: In vivo and in vitro formation of morphinone and morphine-glutathione adduct in guinea pig. J. Pharmacol. Exp. Ther. 1990, 255, 504–510. [Google Scholar] [PubMed]
- Armstrong, S.C.; Cozza, K.L. Pharmacokinetic drug interactions of morphine, codeine, and their derivatives: Theory and clinical reality, part I. Psychosomatics 2003, 44, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Nagamatsu, K.; Inoue, K.; Terao, T.; Toki, S. Effects of glutathione and phenobarbital on the toxicity of codeinone. Biochem. Pharmacol. 1986, 35, 1675–1678. [Google Scholar] [CrossRef]
- Ishida, T.; Yano, M.; Toki, S. In vivo formation of codeinone and morphinone from codeine. Isolation and identification from guinea pig bile. Drug Metab. Dispos. 1991, 19, 895–899. [Google Scholar]
- Ishida, T.; Yano, M.; Toki, S. In vivo formation of codeinone-glutathione adduct: Isolation and identification of a new metabolite in the bile of codeine-treated guinea pig. J. Anal. Toxicol. 1998, 22, 567–572. [Google Scholar] [CrossRef]
- Nagamatsu, K.; Terao, T.; Toki, S. In vitro formation of codeinone from codeine by rat or guinea pig liver homogenate and its acute toxicity in mice. Biochem. Pharmacol. 1985, 34, 3143–3146. [Google Scholar] [CrossRef]
- Pant, K.; Roden, N.; Zhang, C.; Bruce, S.; Wood, C.; Pendino, K. Modified in vivo comet assay detects the genotoxic potential of 14-hydroxycodeinone: An α,β-unsaturated ketone in oxycodone. Environ. Mol. Mutagen. 2015, 56, 777–787. [Google Scholar] [CrossRef]
- Gilliland, R.A.; Möller, C.; DeCaprio, A.P. LC-MS/MS based detection and characterization of covalent glutathione modifications formed by reactive drug of abuse metabolites. Xenobiotica 2019, 49, 778–790. [Google Scholar] [CrossRef] [PubMed]
- Dahlin, D.C.; Miwa, G.T.; Lu, A.Y.; Nelson, S.D. N-acetyl-p-benzoquinone imine; a cytochrome P-450-mediated oxidation product of acetaminophen. Proc. Natl. Acad. Sci. USA 1984, 81, 1327–1331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, C.; Zhong, D.; Chen, X. A fragmentation-based method for the differentiation of glutathione conjugates by high-resolution mass spectrometry with electrospray ionization. Anal. Chim. Acta 2013, 788, 89–98. [Google Scholar] [CrossRef]
- Schneider, K.J.; DeCaprio, A.P. Covalent thiol adducts arising from reactive intermediates of cocaine biotransformation. Chem. Res. Toxicol. 2013, 26, 1755–1764. [Google Scholar] [CrossRef]
- Meyer, M.R.; Richter, L.H.J.; Maurer, H.H. Methylenedioxy designer drugs; mass spectrometric characterization of their glutathione conjugates by means of liquid chromatography-high-resolution mass spectrometry/mass spectrometry and studies on their glutathionyl transferase inhibition potency. Anal. Chim. Acta 2014, 822, 37–50. [Google Scholar] [CrossRef]
- Root-Bernstein, R.; Fewins, J.; Rhinesmith, T.; Koch, A.; Dillon, P.F. Enzymatic recycling of ascorbic acid from dehydroascorbic acid by glutathione-like peptides in the extracellular loops of aminergic G-protein coupled receptors. J. Mol. Recognit. 2016, 29, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Root-Bernstein, R.; Dillon, P.F. Fostering adventure research. A case study of the discovery that ascorbic acid enhances adrenergic drug activity. Drug Dev. Res. 2002, 57, 58–74. [Google Scholar] [CrossRef]
- Dillon, P.F.; Root-Bernstein, R.S.; Lieder, C.M. Antioxidant-independent ascorbate enhancement of catecholamine-induced contractions of vascular smooth muscle. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H2353–H2360. [Google Scholar] [CrossRef]
- Dillon, P.F.; Root-Bernstein, R.; Lieder, C.M. Ascorbate enhancement of H1 histamine receptor sensitivity coincides with ascorbate oxidation inhibition by histamine receptors. Am. J. Physiol. Cell Phys. 2006, 291, C977–C984. [Google Scholar] [CrossRef] [Green Version]
- Dillon, P.F.; Root-Bernstein, R.; Robinson, N.E.; Abraham, W.M.; Berney, C. Receptor-mediated enhancement of beta adrenergic drug activity by ascorbate in vitro and in vivo. PLoS ONE 2010, 5, e15130. [Google Scholar] [CrossRef] [Green Version]
- Shinke, T.; Shite, J.; Takaoka, H.; Hata, K.; Inoue, N.; Yoshikawa, R.; Matsumoto, H.; Masai, H.; Watanabe, S.; Ozawa, T.; et al. Vitamin C restores the contractile response to dobutamine and improves myocardial efficiency in patients with heart failure after anterior myocardial infarction. Am. Heart J. 2007, 154, 645.e1–645.e8. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Berkowitz, B.A. Stereoselective and calcium-dependent contractile effects of narcotic antagonist analgesics in the vascular smooth muscle of the rat. J. Pharmacol. Exp. Ther. 1976, 198, 347–356. [Google Scholar] [PubMed]
- Marti, M.C. The effects of chronic morphine administration to mice on the contractile activity in vitro of the vas deferens without and with morphine. Eur. J. Pharmacol. 1982, 78, 439–447. [Google Scholar] [CrossRef]
- Rae, G.A.; De Moraes, S. Supersensitivity to noradrenaline in vas deferens from morphine-dependent mice is confirmed. Eur. J. Pharmacol. 1983, 86, 347–352. [Google Scholar] [CrossRef]
- Lechner, R.B.; Gurll, N.J.; Reynolds, D.G. Naloxone potentiates the cardiovascular effects of catecholamines in canine hemorrhagic shock. Circ. Shock 1985, 16, 347–361. [Google Scholar]
- Lechner, R.B. Naloxone potentiates inotropic but not chronotropic effects of isoproterenol in vitro. Circ. Shock 1993, 39, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Caffrey, J.L.; Hathorne, L.F.; Carter, G.C.; Sinclair, R.J. Naloxone potentiates contractile responses to epinephrine in isolated canine arteries. Circ. Shock 1990, 31, 317–332. [Google Scholar]
- Caffrey, J.L.; Stoll, S.T.; Sinclair, R.J.; Barron, B.A. (+) naloxone enhances vascular contractile responses to added epinephrine. Prog. Clin. Biol. Res. 1990, 328, 375–378. [Google Scholar]
- Gu, H.; Gaugl, J.F.; Barron, B.A.; Caffrey, J.L. Naloxone enhances cardiac contractile responses to epinephrine without altering epinephrine uptake from plasma. Circ. Shock 1990, 32, 257–271. [Google Scholar]
- He, J.-R.; Molnar, J.; Barraclough, C.A. Morphine amplifies norepinephrine (NE)-induced LH release but blocks NE-stimulated increases in LHRH mRNA levels: Comparison of responses obtained in ovariectomized, estrogen-treated normal and androgen-sterilized rats. Mol. Brain Res. 1993, 20, 71–78. [Google Scholar] [CrossRef]
- Kindman, L.A.; Kates, R.E.; Ginsburg, R. Opioids potentiate contractile response of rabbit myocardium to the beta adrenergic agonist isoproterenol. J. Cardiovasc. Pharmacol. 1991, 17, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Parra, L.; Pérez-Vizcaíno, F.; Alsasua, A.; Martín, M.I.; Tamargo, J. Mu- and delta-opioid receptor-mediated contractile effects on rat aortic vascular smooth muscle. Eur. J. Pharmacol. 1995, 277, 99–105. [Google Scholar] [CrossRef]
- Park, W.K.; Chang, C.H.; Chae, J.E.; Kim, M.H.; Cho, Y.L.; Ahn, D.S. Phosphodiesterase inhibition by naloxone augments the inotropic actions of beta-adrenergic stimulation. Acta Anaesthesiol. Scand. 2009, 53, 1043–1051. [Google Scholar] [CrossRef] [PubMed]
- Root-Bernstein, R.; Dillon, P.F. A common molecular motif characterizes extracellular allosteric enhancers of GPCR aminergic receptors and suggests enhancer mechanism of action. Curr. Med. Chem. 2014, 21, 3673–3686. [Google Scholar] [CrossRef] [Green Version]
- Root-Bernstein, R.; Turke, M.; Subhramanyam, U.K.T.; Churchill, B.; Labahn, J. Adrenergic agonists bind to adrenergic-receptor-like regions of the mu opioid receptor: Enhancing morphine and methionine-enkephalin binding: A new approach to “biased opioids”? Int. J. Mol. Sci. 2018, 19, 272. [Google Scholar] [CrossRef] [Green Version]
- Root-Bernstein, R.; Churchill, B.; Turke, M.; Subhramanyam, U.K.T.; Labahn, J. Mutual enhancement of opioid and adrenergic receptors by combinations of opioids and adrenergic ligands is reflected in molecular complementarity of ligands; Drug development possibilities. Int. J. Mol. Sci. 2019, 20, 4137. [Google Scholar] [CrossRef] [Green Version]
- Root-Bernstein, R.S.; Dillon, P.F.; Hollingsworth, R. A tethered ascorbate-norepinephrine compound, 4-UT, displays long-acting adrenergic activity on rabbit aortic smooth muscle. Drug Res. Dev. 2008, 69, 242–250. [Google Scholar] [CrossRef]
- Buck, M.G.; Zadunaisky, J.A. Stimulation of ion transport by ascorbic acid through inhibition of 3′;5′-cyclic-AMP phosphodiesterase in the corneal epithelium and other tissues. Biochim. Biophys. Acta 1975, 389, 251–260. [Google Scholar] [CrossRef]
- Tisdale, M.J. Inhibition of cyclic adenosine 3′,5′-monophosphate phosphodiesterase from Walker carcinoma by ascorbic and dehydroascorbic acids. Biochem. Biophys. Res. Commun. 1975, 62, 877–882. [Google Scholar] [CrossRef]
- Lewin, S. Hydrolytic rupture of ascorbate by adenosine 3′,5′-cyclic monophosphate phosphodiesterase. Biochem. Soc. Trans. 1976, 4, 71–73. [Google Scholar] [CrossRef] [Green Version]
- Schoepflin, G.S.; Pickett, W.; Austen, K.F.; Goetzl, E.J. Elevation of the cyclic GMP concentration in human platelets by sodium ascorbate and 5-hydroxytryptamine. J. Cyclic Nucleotide Res. 1977, 3, 355–365. [Google Scholar] [PubMed]
- Atkinson, J.P.; Weiss, A.; Ito, M.; Kelly, J.; Parker, C.W. Effects of ascorbic acid and sodium ascorbate on cyclic nucleotide metabolism in human lymphocytes. J. Cyclic Nucleotide Res. 1979, 5, 107–123. [Google Scholar] [PubMed]
- Malamud, D.; Kroll, Y. Ascorbic acid inhibition of cyclic nucleotide phosphodiesterase activity. Proc. Soc. Exp. Biol. Med. 1980, 164, 534–536. [Google Scholar] [CrossRef]
- Puri, S.K.; Cochin, J.; Volicer, L. Effect of morphine sulfate on adenylate cyclase and phosphodiesterase activities in rat corpus striatum. Life Sci. 1975, 16, 759–767. [Google Scholar] [CrossRef]
- Yu, X.C.; Li, H.Y.; Wang, H.X.; Wong, T.M. U50, 488H inhibits effects of norepinephrine in rat cardiomyocytes-cross-talk between kappa-opioid and beta-adrenergic receptors. J. Mol. Cell Cardiol. 1998, 30, 405–413. [Google Scholar]
- Fujita, W.; Gomes, I.; Devi, L.A. Revolution in GPCR signalling: Opioid receptor heteromers as novel therapeutic targets: IUPHAR review 10. Br. J. Pharmacol. 2014, 171, 4155–4176. [Google Scholar] [CrossRef] [Green Version]
- Chabot-Doré, A.J.; Schuster, D.J.; Stone, L.S.; Wilcox, G.L. Analgesic synergy between opioid and α2 -adrenoceptors. Br. J. Pharmacol. 2015, 172, 388–402. [Google Scholar] [CrossRef] [Green Version]
- Visor, G.C.; Lin, L.H.; Kenley, R.A.; Venuti, M.C.; Alvarez, R. Electrochemical evaluation of the interaction between ascorbic acid and the cardiotonic drug RS-82856. Drug Des. Deliv. 1987, 2, 121–128. [Google Scholar]
- Dillon, P.F.; Root-Bernstein, R.; Sears, P.R.; Olson, L.K. Natural electrophoresis of norepinephrine and ascorbic acid. Biophys. J. 2000, 79, 370–376. [Google Scholar] [CrossRef] [Green Version]
- Dillon, P.F.; Root-Bernstein, R.; Lieder, C.M. Molecular shielding of electric field complex dissociation. Biophys. J. 2006, 90, 432–438. [Google Scholar] [CrossRef] [Green Version]
- Root-Bernstein, R.; Dillon, P.F. Molecular complementarity I; the complementarity theory of the origin and evolution of life. J. Theor. Biol. 1997, 188, 447–479. [Google Scholar] [CrossRef] [PubMed]
- Root-Bernstein, R.; Dillon, P.F. Small molecule complementarity as a source of novel pharmaceutical agents and combination therapies. Curr. Pharm. Des. 2008, 14, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Rubenstein, L.A.; Zauhar, R.J.; Lanzara, R.G. Molecular dynamics of a biophysical model for beta2-adrenergic and G protein-coupled receptor activation. J. Mol. Graph. Model 2006, 25, 396–409. [Google Scholar] [CrossRef] [PubMed]
- Root Bernstein, R. Catecholamines bind to enkephalins, morphiceptin, and morphine. Brain Res. Bull. 1987, 18, 509–532. [Google Scholar] [CrossRef]
- Pan, D.Q.; Jiang, M.; Liu, T.T.; Wang, Q.; Shi, J.H. Combined spectroscopies and molecular docking approach to characterizing the binding interaction of enalapril with bovine serum albumin. Luminescence 2017, 32, 481–490. [Google Scholar] [CrossRef]
- Shi, J.H.; Pan, D.Q.; Wang, X.X.; Liu, T.T.; Jiang, M.; Wang, Q. Characterizing the binding interaction between antimalarial artemether (AMT) and bovine serum albumin (BSA); Spectroscopic and molecular docking methods. J. Photochem. Photobiol. B 2016, 162, 14–23. [Google Scholar] [CrossRef]
- Zhang, Y.; Qi, Z.; Zheng, D.; Li, C.; Liu, Y. Interactions of chromium (III) and chromium (VI) with bovine serum albumin studied by UV spectroscopy, circular dichroism, and fluorimetry. Biol. Trace Elem. Res. 2009, 130, 172–184. [Google Scholar] [CrossRef]
- Root-Bernstein, R.; Podufaly, A.; Dillon, P.F. Estradiol binds to insulin and insulin receptor decreasing insulin binding in vitro. Front. Endocrinol. (Lausanne) 2014, 5, 118. [Google Scholar] [CrossRef] [Green Version]
- Moeslinger, T.; Brunner, M.; Volf, I.; Spieckermann, P.G. Spectrophotometric determination of ascorbic acid and dehydroascorbic acid. Clin. Chem. 1995, 41, 1177–1181. [Google Scholar] [CrossRef]
- Seghieri, G.; Martinoli, L.; Di Felici, M.; Anichini, R.; Fazzine, A.; Cuiti, M.; Micheli, M.; Gaspa, L.; Franconi, F. Plasma and platelet ascorbate pools and lipid peroxidation in insulin-dependent diabetes mellitus. Eur. J. Clin. Investig. 1998, 28, 659–663. [Google Scholar] [CrossRef]
- Regulus, P.; Desilets, J.F.; Klarskov, K.; Wagner, J.R. Characterization and detection in cells of a novel adduct derived from the conjugation of glutathione and dehydroascorbate. Free Radic. Biol. Med. 2010, 49, 984–991. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, H.; Giblin, F.J.; Winkler, B.S.; Chakrapani, B.; Leverenz, V.; Shu, C.C. A protective role for glutathione-dependent reduction of dehydroascorbic acid in lens epithelium. Investig. Ophthalmol. Vis. Sci. 1995, 36, 1804–1817. [Google Scholar]
- Heacock, R.A.; Mattok, G.L. The reaction of adrenochrome with glutathione. Arch. Biochem. Biophys. 1964, 107, 352–353. [Google Scholar] [CrossRef]
- Roston, S. Studies of the epinephrine-glutathione reaction in aqueous solution and human blood. Arch Biochem Biophys. 1965, 109, 41–48. [Google Scholar] [CrossRef]
- Silva, R.; Boldt, S.; Costa, V.M.; Carmo, H.; Carvalho, M.; Carvalho, F.; Bastos, M.L.; Lemos-Amado, F.; Remião, F. Evaluation of GSH adducts of adrenaline in biological samples. Biomed. Chromatogr. 2007, 21, 670–679. [Google Scholar] [CrossRef]
- Hiramatsu, M.; Kumagai, Y.; Unger, S.E.; Cho, A.K. Metabolism of methylenedioxymethamphetamine; formation of dihydroxymethamphetamine and a quinone identified as its glutathione adduct. J. Pharmacol. Exp. Ther. 1990, 254, 521–527. [Google Scholar]
- Carvalho, F.; Remião, F.; Amado, F.; Domingues, P.; Correia, A.J.; Bastos, M.L. d-Amphetamine interaction with glutathione in freshly isolated rat hepatocytes. Chem. Res. Toxicol. 1996, 9, 1031–1036. [Google Scholar] [CrossRef]
- Antolino-Lobo, I.; Meulenbelt, J.; Molendijk, J.; Nijmeijer, S.M.; Scherpenisse, P.; van den Berg, M.; van Duursen, M.B. Induction of glutathione synthesis and conjugation by 3.;4-methylenedioxymethamphetamine (MDMA) and 3.;4-dihydroxymethamphetamine (HHMA) in human and rat liver cells.; including the protective role of some antioxidants. Toxicology 2011, 289, 175–184. [Google Scholar] [CrossRef]
- Patel, N.; Kumagai, Y.; Unger, S.E.; Fukuto, J.M.; Cho, A.K. Transformation of dopamine and alpha-methyldopamine by NG108-15 cells: Formation of thiol adducts. Chem. Res. Toxicol. 1991, 4, 421–426. [Google Scholar] [CrossRef]
- Hastings, T.G.; Zigmond, M.J. Identification of catechol-protein conjugates in neostriatal slices incubated with [3H]dopamine; impact of ascorbic acid and glutathione. J. Neurochem. 1994, 63, 1126–1132. [Google Scholar] [CrossRef]
- Moszczynska, A.; Turenne, S.; Kish, S.J. Rat striatal levels of the antioxidant glutathione are decreased following binge administration of methamphetamine. Neurosci. Lett. 1998, 255, 49–52. [Google Scholar] [CrossRef]
- Ito, S.; Mori, T.; Kanazawa, H.; Sawaguchi, T. Differential effects of the ascorbyl and tocopheryl derivative on the methamphetamine-induced toxic behavior and toxicity. Toxicology 2007, 240, 96–110. [Google Scholar] [CrossRef] [PubMed]
- Baez, S.; Segura-Aguilar, J.; Widersten, M.; Johansson, A.S.; Mannervik, B. Glutathione transferases catalyse the detoxication of oxidized metabolites (o-quinones) of catecholamines and may serve as an antioxidant system preventing degenerative cellular processes. Biochem. J. 1997, 324 Pt 1, 25–28. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.W.; Zhang, Z.H.; Wang, R.; Xie, Y.F.; Qiao, J.T.; Dafny, N. Norepinephrine and serotonin-induced antinociception are blocked by naloxone with different dosages. Brain Res. Bull. 1994, 3, 113–117. [Google Scholar]
- Misra, A.L.; Woods, L.A. Evidence for interaction in vitro of morphine with glutathione. Nature 1970, 228, 1226–1227. [Google Scholar] [CrossRef] [PubMed]
- National Highway Traffic Safety Administration. Drugs and Human Performance Fact Sheets. Morphine. Available online: http://www.nhtsa.gov/people/injury/research/job185drugs/morphine.htm (accessed on 13 July 2012).
- Zheng, X.; Zhang, T.; Ding, H.; Wang, C. Plasma levels of β-endorphin, leucine enkephalin and arginine vasopressin in patients with essential hypertension and the effects of clonidine. Int. J. Cardiol. 1995, 51, 233–244. [Google Scholar] [CrossRef]
- Rhoads, D.E.; Sankaran, H.; Peterson, N.A.; Raghupathy, E. Interaction of enkephalins and des-tyrosyl-enkephalins with synaptosomal plasma membrane vesicles: Enkephalin binding and inhibition of proline transport. Biochemistry 1986, 25, 1580–1584. [Google Scholar] [CrossRef]
- Dzubaym, J.A.; Jahr, C.E. The concentration of synaptically released glutamate outside of the climbing fiber-purkinje cell synaptic cleft. J. Neurosci. 1999, 19, 5265–5274. [Google Scholar] [CrossRef]
- Matsui, K.; Jahr, C.E.; Rubio, M.E. High-concentration rapid transients of glutamate mediate neural-glial communication via ectopic release. J. Neurosci. 2005, 25, 7538–7547. [Google Scholar] [CrossRef]
- Moussawi, K.; Riegel, A.; Nair, S.; Kalivas, P.W. Extracellular glutamate: Functional compartments operate in different concentration ranges. Front. Syst. Neurosci. 2011, 5, 94. [Google Scholar] [CrossRef] [Green Version]
- Ogita, K.; Ogawa, Y.; Yoneda, Y. Apparent binding activity of [H]glutathione in rat central and peripheral tissues. Neurochem. Int. 1988, 13, 493–497. [Google Scholar] [CrossRef]
- Liu, Y.F.; Quirion, R. Modulatory role of glutathione on mu-opioid, substance P/neurokinin-1, and kainic acid receptor binding sites. J. Neurochem. 1992, 59, 1024–1032. [Google Scholar] [CrossRef] [PubMed]
- Janáky, R.; Ogita, K.; Pasqualotto, B.A.; Bains, J.S.; Oja, S.S.; Yoneda, Y.; Shaw, C.A. Glutathione and signal transduction in the mammalian CNS. J. Neurochem. 1999, 73, 889–902. [Google Scholar] [CrossRef] [PubMed]
- Kanigel, R. Apprentice to Genius; Macmillan: New York, NY, USA, 1986; pp. 171–201. [Google Scholar]
- Benyhe, S.; Farkas, J.; Tóth, G.; Wollemann, M. Met5-enkephalin-Arg 6-Phe7, an endogenous neuropeptide, binds to multiple opioid and nonopioid sites in rat brain. J. Neurosci. Res. 1998, 48, 249–258. [Google Scholar] [CrossRef]
- Webster, J.L.; Polgar, W.E.; Brandt, S.R.; Berzetei-Gurske, I.P.; Toll, L. Comparison of κ2-opioid receptors in guinea pig brain and guinea pig ileum membranes. Eur. J. Pharmacol. 1992, 231, 251–258. [Google Scholar] [CrossRef]
- Munro, T.A.; Huang, X.-P.; Inglese, C.; Perrone, M.G.; Van’t Veer, A.; Carroll, F.I.; Béguin, C.; Carlezon, W.A., Jr.; Colabufo, N.A.; Cohen, B.M. Selective κ opioid antagonists nor-BNI.; GNTI and JDTic have low affinities for non-opioid receptors and transporters. PLoS ONE 2013, 8, e70701. [Google Scholar] [CrossRef] [Green Version]
- Root-Bernstein, R. Adrenergic agonists and the mu opioid receptor. In The Neurobiology, Physiology, and Behavior of Pain; Preedy, Ed.; Elsevier: Amsterdam, The Netherlands; New York, NY, USA, 2020; in press. [Google Scholar]
- Zhang, Y.T.; Zheng, Q.S.; Pan, J.; Zheng, R.L. Oxidative damage of biomolecules in mouse liver induced by morphine and protected by antioxidants. Basic Clin. Pharmacol. Toxicol. 2004, 95, 53–58. [Google Scholar] [CrossRef]
- Ayatollahi, V.; Dehghanpour Farashah, S.; Behdad, S.; Vaziribozorg, S.; Rabbani Anari, M. Effect of intravenous vitamin C on postoperative pain in uvulopalatopharyngoplasty with tonsillectomy. Clin. Otolaryngol. 2017, 42, 139–143. [Google Scholar] [CrossRef]
- Laflı Tunay, D.; Türkeün Ilgınel, M.; Ünlügenç, H.; Tunay, M.; Karacaer, F.; Biricik, E. Comparison of the effects of preoperative melatonin or vitamin C administration on postoperative analgesia. Bosn. J. Basic Med. Sci. 2020, 20, 117–124. [Google Scholar] [CrossRef] [Green Version]
- Chaitanya, N.C.; Muthukrishnan, A.; Krishnaprasad, C.M.S.; Sanjuprasanna, G.; Pillay, P.; Mounika, B. An insight and update on the analgesic properties of vitamin C. J. Pharm. Bioallied. Sci. 2018, 10, 119–125. [Google Scholar] [CrossRef]
- Pinkerton, E.; Good, P.; Gibbons, K.; Hardy, J. An open-label pilot study of oral vitamin C as an opioid-sparing agent in patients with chronic pain secondary to cancer. Support Care Cancer 2017, 25, 341–343. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Roffey, D.M.; Dion, C.A.; Arab, A.; Wai, E.K. Effect of perioperative vitamin C supplementation on postoperative pain and the incidence of chronic regional pain syndrome: A systematic review and meta-analysis. Clin. J. Pain 2016, 32, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.; Park, J.S.; Moon, S.; Yeo, J. Effect of intravenous high dose vitamin C on postoperative pain and morphine use after laparoscopic colectomy: A randomized controlled trial. Pain Res. Manag. 2016, 2016, 9147279. [Google Scholar] [CrossRef] [PubMed]
- Evangelou, A.; Kalfakakou, V.; Georgakas, P.; Koutras, V.; Vezyraki, P.; Iliopoulou, L.; Vadalouka, A. Ascorbic acid (vitamin C) effects on withdrawal syndrome of heroin abusers. In Vivo 2000, 14, 363–366. [Google Scholar] [PubMed]
- Johnston, P.A.; Chahl, L.A. Chronic treatment with ascorbic acid inhibits the morphine withdrawal response in guinea-pigs. Neurosci. Lett. 1992, 135, 23–27. [Google Scholar] [CrossRef]
- Khanna, N.C.; Sharma, S.K. Megadoses of vitamin C prevent the development of tolerance and physical dependence on morphine in mice. Life Sci. 1983, 33 (Suppl. 1), 401–404. [Google Scholar] [CrossRef]
- Park, H.S.; Kim, J.H.; Kim, Y.J.; Kim, D.Y. Plasma concentrations of morphine during postoperative pain control. Korean J. Pain. 2011, 24, 146–153. [Google Scholar] [CrossRef]
- Collins, S.L.; Faura, C.C.; Moore, R.A.; McQuay, H.J. Peak plasma concentrations after oral morphine: A systematic review. J. Pain Symptom Manag. 1998, 16, 388–402. [Google Scholar] [CrossRef]
- Sjöström, S.; Tamsen, A.; Persson, M.P.; Hartvig, P. Pharmacokinetics of intrathecal morphine and meperidine in humans. Anesthesiology 1987, 67, 889–895. [Google Scholar] [CrossRef]
- Jenkins, A.J.; Oyler, J.M.; Cone, E.J. Comparison of heroin and cocaine concentrations in saliva with concentrations in blood and plasma. J. Anal. Toxicol. 1995, 19, 359–374. [Google Scholar] [CrossRef]
- Dubois, N.; Demaret, I.; Ansseau, M.; Rozet, E.; Hubert, P.; Charlier, C. Plasma level monitoring of the major metabolites of diacetylmorphine (heroin) by the “chasing the dragon” route in severe heroin addicts. Acta Clin. Belg. 2013, 68, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Haghbin, M.A.; Navidi, Z.; Romero-Leguizamon, C.R.; Shabani, M. Morphine in plasma and cerebrospinal fluid of patients addicted to opiates undergoing surgery: High-performance liquid chromatography method. Addict. Health 2018, 10, 95–101. [Google Scholar] [CrossRef] [PubMed]







| Kd (µM) | Glut RED ECG | Glut OX ECG-ECG | Mu OPR 38–51 | Mu OPR 111–122 | Mu OPR 121–131 | Mu OPR 132–143 | Mu OPR 211–226 | INSR 157–166 | INSR 392–404 |
|---|---|---|---|---|---|---|---|---|---|
| Ascorbic Acid | 60 | 20/310 | 70 | >1000 | >1000 | 5/40 | 65/700 | 100 | >1000 |
| Dehydro Asc | 60 | 60 | >1000 | >1000 | >1000 | >1000 | 150 | 150 | >1000 |
| Glucose | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 |
| Morphine | 60 | 300 | 35 | 50 | 900 | 35 | 30 | 60 | >1000 |
| Methadone | 60 | 300 | 70 | 70 | >1000 | 50 | 150 | 80 | >1000 |
| Naloxone | 50 | 300 | 0.5/35 | 0.5/38 | >1000 | 0.5/42 | 1.0/45 | 200 | >1000 |
| Met-Enkephalin | 70 | 400 | 1.2/35 | 0.33/80 | 3.5/90 | 0.4/70 | 1.0/65 | 100 | >1000 |
| Phenylephrine | 5 | 12 | 1.0/30 | 150 | >1000 | 60 | 150 | 70 | >1000 |
| Propranolol | 5 | 9 | 25 | 70 | >1000 | 90 | 200 | 300 | 700 |
| Amphetamine | 20 | 60 | 1.3/90 | 1.3/100 | >1000 | 1.1/85 | 1.2/90 | 400 | >1000 |
| Epinephrine | 40 | 63 | 1.2/35 | 1.3/40 | >1000 | 1.4/35 | 1.2/45 | >1000 | 200 |
| Norepinephrine | 110 | 60 | 1.4/45 | 1.3/40 | >1000 | 1.4/40 | 1.3/45 | 140 | >1000 |
| Dopamine | 90 | 90 | 60 | 65 | >1000 | 60 | 65 | 400 | >1000 |
| L-DOPA | 50 | 50 | 80 | 60 | >1000 | 70 | 150 | 90 | >1000 |
| Tyrosine | 130 | 130 | 85 | 700 | >1000 | 60 | 160 | 90 | >1000 |
| Phenylalanine | >1000 | 90 | 50 | >1000 | >1000 | 80 | 200 | 90 | >1000 |
| Histamine | 500 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | 110 | >1000 |
| Serotonin | 70 | >1000 | 100 | 100 | 350 | 100 | 90 | 110 | 900 |
| Melatonin | 100 | >1000 | 50 | 400 | >1000 | 65 | 130 | 80 | >1000 |
| Acetylcholine | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 |
| Glutamate | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 |
| Glycine | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 |
| Kd (µM) | B2AR 97–103 KMWTFGN | B2AR 105–108 WCEF | B2AR 183–185 NCY | D1DR 89–100 FWPFGSFCN | D1DR 96–98 FCN | DRD1 177–188 ATSLAETINCDS | H1HR 77–87 GAVVMPMNILYL | H1HR 105–108 SMDY | HIHR 177–183 RDKCETD |
|---|---|---|---|---|---|---|---|---|---|
| Ascorbic Acid | 65 | 35 | 12 | >1000 | 7 | 300 | 300 | 60 | 130 |
| Dehydro Asc | 60 | 20 | 500 | >1000 | 20 | 200 | 35 | 15 | >1000 |
| Glucose | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | 60 | >1000 | >1000 |
| Morphine | 1 | 30 | >1000 | 30 | 25 | 310 | 110 | 50 | 30 |
| Methadone | 300 | 30 | >1000 | 120 | 25 | 110 | 50 | 10 | 150 |
| Naloxone | 6 | 30 | >1000 | 60 | 30 | 150 | 110 | 50 | 40 |
| Met-Enkephalin | 130 | 30 | >1000 | 10 | 30 | 150 | 2.3/70 | 3.0/70 | 55 |
| Phenylephrine | >1000 | 35 | 30 | >1000 | 50 | 80 | >1000 | 50 | 50 |
| Propranolol | >1000 | 40 | 22 | >1000 | 20 | 80 | >1000 | 45 | 50 |
| Amphetamine | 130 | 20 | 2.3 | 530 | 2.5 | 230 | 60 | 70 | 35 |
| Epinephrine | 120 | 35 | 25 | 400 | 40 | 900 | 30 | 60 | 40 |
| Norepinephrine | 600 | 35 | 12 | 300 | 50 | 1000 | 30 | 30 | 45 |
| Dopamine | >1000 | 30 | 35 | 750 | 50 | 300 | 30 | 35 | 50 |
| L-DOPA | 200 | 40 | 25 | >1000 | 60 | 210 | 90 | 35 | 55 |
| Tyrosine | >1000 | 50 | 35 | >1000 | 22 | 80 | >1000 | 50 | 50 |
| Phenylalanine | >1000 | 55 | 30 | >1000 | 25 | 130 | >1000 | 60 | 50 |
| Histamine | >1000 | 50 | >1000 | 600 | 50 | >1000 | 20 | 70 | 70 |
| Serotonin | 430 | 45 | >1000 | >1000 | 30 | >1000 | 50 | 120 | 60 |
| Melatonin | 600 | 25 | >1000 | 750 | 22 | 130 | 55 | 50 | 55 |
| Acetylcholine | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 |
| Glutamate | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 |
| Glycine | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 |
| BINDING ^ | Ascorbate | Opioids | Adrenergics | Serotonin Melatonin | Histamine | Acetyl-Choline | Glutamate | Glucose |
|---|---|---|---|---|---|---|---|---|
| Glutathione | YES/no | YES/no | YES/YES | no/no | no/no | no/no | no/no | no/no |
| Opioid Receptor | YES/YES | YES/YES | YES/YES | no/no | no/no | no/no | no/no | no/no |
| Adrenergic Receptor | YES/YES | YES/YES | YES/YES | YES/no | YES/no | no/no | no/no | no/no |
| Dopamine Receptor | YES/YES | YES/no | YES/YES | YES/no | YES/no | no/no | no/no | no/no |
| Histamine Receptor | YES/no | YES/YES | YES/no | YES/no | YES/YES | no/no | no/no | no/no |
| Insulin Receptor | no/no | no/no | no/no | no/no | no/no | no/no | no/no | no/no |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Root-Bernstein, R.; Churchill, B.; Turke, M. Glutathione and Glutathione-Like Sequences of Opioid and Aminergic Receptors Bind Ascorbic Acid, Adrenergic and Opioid Drugs Mediating Antioxidant Function: Relevance for Anesthesia and Abuse. Int. J. Mol. Sci. 2020, 21, 6230. https://doi.org/10.3390/ijms21176230
Root-Bernstein R, Churchill B, Turke M. Glutathione and Glutathione-Like Sequences of Opioid and Aminergic Receptors Bind Ascorbic Acid, Adrenergic and Opioid Drugs Mediating Antioxidant Function: Relevance for Anesthesia and Abuse. International Journal of Molecular Sciences. 2020; 21(17):6230. https://doi.org/10.3390/ijms21176230
Chicago/Turabian StyleRoot-Bernstein, Robert, Beth Churchill, and Miah Turke. 2020. "Glutathione and Glutathione-Like Sequences of Opioid and Aminergic Receptors Bind Ascorbic Acid, Adrenergic and Opioid Drugs Mediating Antioxidant Function: Relevance for Anesthesia and Abuse" International Journal of Molecular Sciences 21, no. 17: 6230. https://doi.org/10.3390/ijms21176230
APA StyleRoot-Bernstein, R., Churchill, B., & Turke, M. (2020). Glutathione and Glutathione-Like Sequences of Opioid and Aminergic Receptors Bind Ascorbic Acid, Adrenergic and Opioid Drugs Mediating Antioxidant Function: Relevance for Anesthesia and Abuse. International Journal of Molecular Sciences, 21(17), 6230. https://doi.org/10.3390/ijms21176230