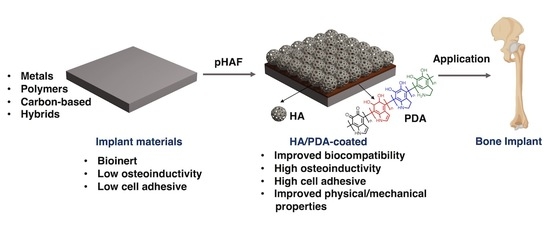
Strategies for Using Polydopamine to Induce Biomineralization of Hydroxyapatite on Implant Materials for Bone Tissue Engineering
Abstract
:1. Introduction
2. Polydopamine Hydroxyapatite Functionalization
2.1. Polydopamine
2.2. Hydroxyapatite Biomaterials and Biomineralization
2.3. Functionalization Process
3. Molecular Interaction of PDA or HA with Biological Cells
4. Application of PDA-HA Functionalization for Implant Materials
4.1. Metals
4.1.1. Titanium-Based Materials
4.1.2. Magnesium-Based Materials
4.1.3. Calcium-Based Materials
4.2. Polymers
4.2.1. Synthetic Polymers
4.2.2. Natural Polymers
4.3. Carbon-Based Materials
5. Conclusion and Future Prospectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| PDA | Polydopamine |
| HA | Hydroxyapatite |
| pHAF | Polydopamine-hydroxyapatite functionalization |
| SBF | Simulated body fluid |
References
- Koons, G.L.; Diba, M.; Mikos, A.G. Materials design for bone-tissue engineering. Nat. Rev. Mater. 2020, 5, 584–603. [Google Scholar] [CrossRef]
- Ryu, J.; Ku, S.H.; Lee, H.; Park, C.B. Mussel-inspired polydopamine coating as a universal route to hydroxyapatite crystallization. Adv. Funct. Mater. 2010, 20, 2132–2139. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-Inspired Surface Chemistry for Multifunctional Coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Ai, K.; Lu, L. Polydopamine and its derivative materials: Synthesis and promising applications in energy, environmental, and biomedical fields. Chem. Rev. 2014, 114, 5057–5115. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.H.; Messersmith, P.B.; Lee, H. Polydopamine Surface Chemistry: A Decade of Discovery. ACS Appl. Mater. Interfaces 2018, 10, 7523–7540. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Li, L.; Li, J.; Yang, C.; Frenkel, N.; Welle, A.; Heissler, S.; Nefedov, A.; Grunze, M.; Levkin, P.A. Uv-triggered dopamine polymerization: Control of polymerization, surface coating, and photopatterning. Adv. Mater. 2014, 26, 8029–8033. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Lu, X.; Liu, K.; Wang, K.; Fang, L.; Weng, L.T.; Zhang, H.; Tang, Y.; Ren, F.; Zhao, C.; et al. Mussel-Inspired Adhesive and Tough Hydrogel Based on Nanoclay Confined Dopamine Polymerization. ACS Nano 2017, 11, 2561–2574. [Google Scholar] [CrossRef]
- Han, L.; Lu, X.; Wang, M.; Gan, D.; Deng, W.; Wang, K.; Fang, L.; Liu, K.; Chan, C.W.; Tang, Y.; et al. A Mussel-Inspired Conductive, Self-Adhesive, and Self-Healable Tough Hydrogel as Cell Stimulators and Implantable Bioelectronics. Small 2017, 13, 1601916. [Google Scholar] [CrossRef]
- Poinard, B.; Kamaluddin, S.; Tan, A.Q.Q.; Neoh, K.G.; Kah, J.C.Y. Polydopamine Coating Enhances Mucopenetration and Cell Uptake of Nanoparticles. ACS Appl. Mater. Interfaces 2019, 11, 4777–4789. [Google Scholar] [CrossRef]
- Zhu, Z.; Su, M. Polydopamine Nanoparticles for Combined Chemo- and Photothermal Cancer Therapy. Nanomaterials 2017, 7, 160. [Google Scholar] [CrossRef]
- Black, K.C.; Yi, J.; Rivera, J.G.; Zelasko-Leon, D.C.; Messersmith, P.B. Polydopamine-enabled surface functionalization of gold nanorods for cancer cell-targeted imaging and photothermal therapy. Nanomedicine 2013, 8, 17–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Cao, J.; Li, H.; Li, J.; Jin, Q.; Ren, K.; Ji, J. Mussel-inspired polydopamine: A biocompatible and ultrastable coating for nanoparticles in vivo. ACS Nano 2013, 7, 9384–9395. [Google Scholar] [CrossRef] [PubMed]
- Mrowczynski, R. Polydopamine-Based Multifunctional (Nano) materials for Cancer Therapy. ACS Appl. Mater. Interfaces 2018, 10, 7541–7561. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zeng, G.; Wang, K.; Wan, Q.; Tao, L.; Zhang, X.; Wei, Y. Recent developments in polydopamine: An emerging soft matter for surface modification and biomedical applications. Nanoscale 2016, 8, 16819–16840. [Google Scholar] [CrossRef]
- Li, L.; Smitthipong, W.; Zeng, H. Mussel-inspired hydrogels for biomedical and environmental applications. Polym. Chem. 2015, 6, 353–358. [Google Scholar] [CrossRef]
- Lynge, M.E.; van der Westen, R.; Postma, A.; Städler, B. Polydopamine—A nature-inspired polymer coating for biomedical science. Nanoscale 2011, 3, 4916. [Google Scholar] [CrossRef]
- Mai, Q.L.; Yiu-Wing, M. Biomaterials for Implants and Scafolds; Springer: Heidelberg, Germany, 2017; ISBN 9783662535721. [Google Scholar]
- Kumar, A.; Kargozar, S.; Baino, F.; Han, S.S. Additive Manufacturing Methods for Producing Hydroxyapatite and Hydroxyapatite-Based Composite Scaffolds: A Review. Front. Mater. 2019, 6, 313. [Google Scholar] [CrossRef]
- Godley, R.; Starosvetsky, D.; Gotman, I. Bonelike apatite formation on niobium metal treated in aqueous NaOH. J. Mater. Sci. Mater. Med. 2004, 15, 1073–1077. [Google Scholar] [CrossRef]
- Liang, K.; Wang, R.; Boutter, M.; Doherty, C.M.; Mulet, X.; Richardson, J.J. Biomimetic mineralization of metal-organic frameworks around polysaccharides. Chem. Commun. 2017, 53, 1249–1252. [Google Scholar] [CrossRef]
- Spoerke, E.D.; Anthony, S.G.; Stupp, S.I. Enzyme directed templating of artificial bone mineral. Adv. Mater. 2009, 21, 425–430. [Google Scholar] [CrossRef] [Green Version]
- Murphy, W.L.; Mooney, D.J. Bioinspired growth of crystalline carbonate apatite on biodegradable polymer substrata. J. Am. Chem. Soc. 2002, 124, 1910–1917. [Google Scholar] [CrossRef] [PubMed]
- Meldrum, F.C.; Colfen, H. Controlling Mineral Morphologies and Structures in Biological and Synthetic Systems. Chem. Rev. 2008, 108, 4332–4432. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, T.; Kushitani, H.; Sakka, S.; Kitsugi, T.; Yamamuro, T. Solutions able to reproduce in vivo surface-structure changes in bioactive glass-ceramic A-W3. J. Biomed. Mater. Res. 1990, 24, 721–734. [Google Scholar] [CrossRef] [PubMed]
- Ohtsuki, C.; Kushitani, H.; Kokubo, T.; Kotani, S.; Yamamuro, T. Apatite formation on the surface of ceravital-type glass-ceramic in the body. J. Biomed. Mater. Res. 1991, 25, 1363–1370. [Google Scholar] [CrossRef]
- Chavan, P.N.; Bahir, M.M.; Mene, R.U.; Mahabole, M.P.; Khairnar, R.S. Study of nanobiomaterial hydroxyapatite in simulated body fluid: Formation and growth of apatite. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2010, 168, 224–230. [Google Scholar] [CrossRef]
- Ku, S.H.; Ryu, J.; Hong, S.K.; Lee, H.; Park, C.B. General functionalization route for cell adhesion on non-wetting surfaces. Biomaterials 2010, 31, 2535–2541. [Google Scholar] [CrossRef]
- Wang, Z.; Li, P.; Jiang, Y.; Jia, Z.; Tang, P.; Lu, X.; Ren, F.; Wang, K.; Yuan, H. Mussel-inspired nanostructured coatings assembled using polydopamine nanoparticles and hydroxyapatite nanorods for biomedical applications. Biosurface Biotribol. 2017, 3, 1–10. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, B.; Jia, Z.; Lu, X.; Fang, L.; Wang, K.; Ren, F. Polydopamine mediated assembly of hydroxyapatite nanoparticles and bone morphogenetic protein-2 on magnesium alloys for enhanced corrosion resistance and bone regeneration. J. Biomed. Mater. Res. Part A 2017, 105, 2750–2761. [Google Scholar] [CrossRef]
- Zhou, T.; Yan, L.; Xie, C.; Li, P.; Jiang, L.; Fang, J.; Zhao, C.; Ren, F.; Wang, K.; Wang, Y.; et al. A Mussel-Inspired Persistent ROS-Scavenging, Electroactive, and Osteoinductive Scaffold Based on Electrochemical-Driven In Situ Nanoassembly. Small 2019, 15, 1805440. [Google Scholar] [CrossRef]
- Xie, C.; Lu, X.; Wang, K.; Yuan, H.; Fang, L.; Zheng, X.; Chan, C.; Ren, F.; Zhao, C. Pulse Electrochemical Driven Rapid Layer-by-Layer Assembly of Polydopamine and Hydroxyapatite Nanofilms via Alternative Redox in Situ Synthesis for Bone Regeneration. ACS Biomater. Sci. Eng. 2016, 2, 920–928. [Google Scholar] [CrossRef]
- Ghorbani, F.; Zamanian, A.; Behnamghader, A.; Joupari, M.D. A facile method to synthesize mussel-inspired polydopamine nanospheres as an active template for in situ formation of biomimetic hydroxyapatite. Mater. Sci. Eng. C 2019, 94, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, F.; Zamanian, A.; Behnamghader, A.; Daliri-Joupari, M. Bone-like hydroxyapatite mineralization on the bio-inspired PDA nanoparticles using microwave irradiation. Surf. Interfaces 2019, 15, 38–42. [Google Scholar] [CrossRef]
- Morais, J.M.; Papadimitrakopoulos, F.; Burgess, D.J. Biomaterials/Tissue Interactions: Possible Solutions to Overcome Foreign Body Response. AAPS J. 2010, 12, 188–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gasik, M. Understanding biomaterial-tissue interface quality: Combined in vitro evaluation. Sci. Technol. Adv. Mater. 2017, 18, 550–562. [Google Scholar] [CrossRef] [Green Version]
- Sinha, R.; Hoon, M.; Baudin, J.; Okawa, H.; Wong, R.O.L.; Rieke, F. Cellular and Circuit Mechanisms Shaping the Perceptual Properties of the Primate Fovea. Cell 2017, 168, 413–426.e12. [Google Scholar] [CrossRef] [Green Version]
- Andalib, M.N.; Dzenis, Y.; Donahue, H.J.; Lim, J.Y. Biomimetic substrate control of cellular mechanotransduction. Biomater. Res. 2016, 20, 11. [Google Scholar] [CrossRef] [Green Version]
- Zhong, S.; Luo, R.; Wang, X.; Tang, L.; Wu, J.; Wang, J.; Huang, R.; Sun, H.; Huang, N. Effects of polydopamine functionalized titanium dioxide nanotubes on endothelial cell and smooth muscle cell. Colloids Surf. B Biointerfaces 2014, 116, 553–560. [Google Scholar] [CrossRef]
- Lee, J.-J.; Park, I.-S.; Shin, G.-S.; Lyu, S.-K.; Ahn, S.-G.; Bae, T.-S.; Lee, M.-H. Effects of polydopamine coating on the bioactivity of titanium for dental implants. Int. J. Precis. Eng. Manuf. 2014, 15, 1647–1655. [Google Scholar] [CrossRef]
- Zhao, M.-H.; Chen, X.-P.; Wang, Q. Wetting failure of hydrophilic surfaces promoted by surface roughness. Sci. Rep. 2014, 4, 5376. [Google Scholar] [CrossRef]
- Al Qahtani, W.M.; Schille, C.; Spintzyk, S.; Al Qahtani, M.S.; Engel, E.; Geis-Gerstorfer, J.; Rupp, F.; Scheideler, L. Effect of surface modification of zirconia on cell adhesion, metabolic activity and proliferation of human osteoblasts. Biomed. Eng./Biomed. Tech. 2017, 62, 75–87. [Google Scholar] [CrossRef]
- Gittens, R.A.; McLachlan, T.; Olivares-Navarrete, R.; Cai, Y.; Berner, S.; Tannenbaum, R.; Schwartz, Z.; Sandhage, K.H.; Boyan, B.D. The effects of combined micron-/submicron-scale surface roughness and nanoscale features on cell proliferation and differentiation. Biomaterials 2011, 32, 3395–3403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ko, E.; Lee, J.S.; Kim, H.; Yang, S.Y.; Yang, D.; Yang, K.; Lee, J.; Shin, J.; Yang, H.S.; Ryu, W.; et al. Electrospun Silk Fibroin Nanofibrous Scaffolds with Two-Stage Hydroxyapatite Functionalization for Enhancing the Osteogenic Differentiation of Human Adipose-Derived Mesenchymal Stem Cells. ACS Appl. Mater. Interfaces 2018, 10, 7614–7625. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Li, H.; Wu, J.; Yang, Q.; Jiang, D.; Qiao, B. Polydopamine-induced hydroxyapatite coating facilitates hydroxyapatite / polyamide 66 implant osteogenesis: An in vitro and in vivo evaluation. Int. J. Nanomed. 2018, 13, 8179–8193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishnaevsky, L.; Levashov, E.; Valiev, R.Z.; Segurado, J.; Sabirov, I.; Enikeev, N.; Prokoshkin, S.; Solov’Yov, A.V.; Korotitskiy, A.; Gutmanas, E.; et al. Nanostructured titanium-based materials for medical implants: Modeling and development. Mater. Sci. Eng. R Rep. 2014, 81, 1–19. [Google Scholar] [CrossRef]
- Lee, J.H.; Lim, Y.W.; Kwon, S.Y.; Kim, Y.S. In vitro effects of mussel-inspired polydopamine coating on Ti6Al4V alloy. Tissue Eng. Regen. Med. 2013, 10, 273–278. [Google Scholar] [CrossRef]
- Ku, S.H.; Park, C.B. Human endothelial cell growth on mussel-inspired nanofiber scaffold for vascular tissue engineering. Biomaterials 2010, 31, 9431–9437. [Google Scholar] [CrossRef]
- Wu, C.; Fan, W.; Chang, J.; Xiao, Y. Mussel-inspired porous SiO2 scaffolds with improved mineralization and cytocompatibility for drug delivery and bone tissue engineering. J. Mater. Chem. 2011, 21, 18300–18307. [Google Scholar] [CrossRef] [Green Version]
- Zhe, W.; Dong, C.; Sefei, Y.; Dawei, Z.; Kui, X.; Xiaogang, L. Facile incorporation of hydroxyapatite onto an anodized Ti surface via a mussel inspired polydopamine coating. Appl. Surf. Sci. 2016, 378, 496–503. [Google Scholar] [CrossRef]
- Wu, M.; Wang, T.; Zhang, J.; Qian, H.; Miao, R.; Yang, X. PDA/CPP bilayer prepared via two-step immersion for accelerating the formation of a crack-free biomimetic hydroxyapatite coating on titanium substrates. Mater. Lett. 2017, 206, 56–59. [Google Scholar] [CrossRef]
- Chien, C.Y.; Tsai, W.B. Poly(dopamine)-assisted immobilization of Arg-Gly-Asp peptides, hydroxyapatite, and bone morphogenic protein-2 on titanium to improve the osteogenesis of bone marrow stem cells. ACS Appl. Mater. Interfaces 2013, 5, 6975–6983. [Google Scholar] [CrossRef]
- Shen, T.; Yang, W.; Shen, X.; Chen, W.; Tao, B.; Yang, X.; Yuan, J.; Liu, P.; Cai, K. Polydopamine-Assisted Hydroxyapatite and Lactoferrin Multilayer on Titanium for Regulating Bone Balance and Enhancing Antibacterial Property. ACS Biomater. Sci. Eng. 2018, 4, 3211–3223. [Google Scholar] [CrossRef]
- Nagano-Takebe, F.; Miyakawa, H.; Nakazawa, F.; Endo, K. Inhibition of initial bacterial adhesion on titanium surfaces by lactoferrin coating. Biointerphases 2014, 9, 029006. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Jiang, P.; Ge, Y.; Lan, F.; Zhou, X.; He, J.; Wu, Y. Dopamine self-polymerized along with hydroxyapatite onto the preactivated titanium percutaneous implants surface to promote human gingival fibroblast behavior and antimicrobial activity for biological sealing. J. Biomater. Appl. 2018, 32, 1071–1082. [Google Scholar] [CrossRef]
- Li, Y.; Yang, W.; Li, X.X.; Zhang, X.; Wang, C.C.C.C.; Meng, X.; Pei, Y.; Fan, X.; Lan, P.; Wang, C.C.C.C.; et al. Improving Osteointegration and Osteogenesis of Three-Dimensional Porous Ti6Al4V Scaffolds by Polydopamine-Assisted Biomimetic Hydroxyapatite Coating. ACS Appl. Mater. Interfaces 2015, 7, 5715–5724. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Ma, X.; Chang, L.; Zhu, S.; Guan, S. Characterization and cytocompatibility of polydopamine on MAO-HA coating supported on Mg-Zn-Ca alloy. Surf. Interface Anal. 2017, 49, 1115–1123. [Google Scholar] [CrossRef]
- Lin, B.; Zhong, M.; Zheng, C.; Cao, L.; Wang, D.; Wang, L.; Liang, J.; Cao, B. Preparation and characterization of dopamine-induced biomimetic hydroxyapatite coatings on the AZ31 magnesium alloy. Surf. Coat. Technol. 2015, 281, 82–88. [Google Scholar] [CrossRef]
- Liu, Z.; Qu, S.; Zheng, X.; Xiong, X.; Fu, R.; Tang, K.; Zhong, Z.; Weng, J. Effect of polydopamine on the biomimetic mineralization of mussel-inspired calcium phosphate cement in vitro. Mater. Sci. Eng. C 2014, 44, 44–51. [Google Scholar] [CrossRef]
- Dawood, A.E.; Parashos, P.; Wong, R.H.K.; Reynolds, E.C.; Manton, D.J. Calcium silicate-based cements: Composition, properties, and clinical applications. J. Investig. Clin. Dent. 2017, 8, e12195. [Google Scholar] [CrossRef]
- Saghiri, M.A.; Orangi, J.; Asatourian, A.; Gutmann, J.L.; Garcia-Godoy, F.; Lotfi, M.; Sheibani, N. Calcium silicate-based cements and functional impacts of various constituents. Dent. Mater. J. 2017, 36, 8–18. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.; Wang, T.; Wang, Q.; Huang, W. Preparation of bio-inspired polydopamine coating on hydrated tricalcium silicate substrate to accelerate hydroxyapatite mineralization. Mater. Lett. 2019, 236, 120–123. [Google Scholar] [CrossRef]
- Wu, M.; Wang, T.; Wang, Y.; Wang, H. Ultrafast bone-like apatite formation on bioactive tricalcium silicate cement using mussel-inspired polydopamine. Ceram. Int. 2019, 45, 3033–3043. [Google Scholar] [CrossRef]
- Lin, H.; Fu, Y.; Gao, Y.; Mo, A. Integrated Design of a Mussel-Inspired Hydrogel Biofilm Composite Structure to Guide Bone Regeneration. Macromol. Mater. Eng. 2020, 305, 2000064. [Google Scholar] [CrossRef]
- Kim, S.; Park, C.B. Mussel-inspired transformation of CaCO3to bone minerals. Biomaterials 2010, 31, 6628–6634. [Google Scholar] [CrossRef] [PubMed]
- Teo, A.J.T.; Mishra, A.; Park, I.; Kim, Y.J.; Park, W.T.; Yoon, Y.J. Polymeric Biomaterials for Medical Implants and Devices. ACS Biomater. Sci. Eng. 2016, 2, 454–472. [Google Scholar] [CrossRef]
- Yun, Y.J.; Kim, H.J.; Lee, D.W.; Um, S.; Chun, H.J. Polydopamine-mediated surface modifications of poly L-lactic acid with hydroxyapatite, heparin and bone morphogenetic protein-2 and their effects on osseointegration. J. Ind. Eng. Chem. 2018, 67, 244–254. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, L.; Liu, H.; Wen, W.; Li, H.; Zhou, C.; Luo, B. Biomineralization guided by polydopamine-modifed poly(L-lactide) fibrous membrane for promoted osteoconductive activity. Biomed. Mater. 2019, 14. [Google Scholar] [CrossRef]
- Liu, C.; Li, Y.; Wang, J.; Liu, C.; Liu, W.; Jian, X. Improving Hydrophilicity and Inducing Bone-Like Apatite Formation on PPBES by Polydopamine Coating for Biomedical Application. Molecules 2018, 23, 1643. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.S.; Jin, Y.; Park, H.J.; Yang, K.; Lee, M.S.; Yang, H.S.; Cho, S.W. In Situ Bone Tissue Engineering With an Endogenous Stem Cell Mobilizer and Osteoinductive Nanofibrous Polymeric Scaffolds. Biotechnol. J. 2017, 12, 1700062. [Google Scholar] [CrossRef]
- Wei, P.F.; Yuan, Z.Y.; Jing, W.; Guan, B.B.; Liu, Z.H.; Zhang, X.; Mao, J.P.; Chen, D.F.; Cai, Q.; Yang, X.P. Regenerating infected bone defects with osteocompatible microspheres possessing antibacterial activity. Biomater. Sci. 2019, 7, 272–286. [Google Scholar] [CrossRef]
- Gan, D.; Wang, Z.; Xie, C.; Wang, X.; Xing, W.; Ge, X.; Yuan, H.; Wang, K.; Tan, H.; Lu, X. Mussel-Inspired Tough Hydrogel with In Situ Nanohydroxyapatite Mineralization for Osteochondral Defect Repair. Adv. Healthc. Mater. 2019, 8, 1901103. [Google Scholar] [CrossRef]
- Tapsir, Z.; Saidin, S. Synthesis and characterization of collagen-hydroxyapatite immobilized on polydopamine grafted stainless steel. Surf. Coat. Technol. 2016, 285, 11–16. [Google Scholar] [CrossRef]
- Li, M.; Liu, X.; Xu, Z.; Yeung, K.W.K.; Wu, S. Dopamine Modified Organic-Inorganic Hybrid Coating for Antimicrobial and Osteogenesis. ACS Appl. Mater. Interfaces 2016, 8, 33972–33981. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, J.; Ban, L.; Qiu, L.; Chen, J.; Zhu, Z.; Wan, Y. Polydopamine regulated hydroxyapatite microspheres grown in the three-dimensional honeycomb-like mollusk shell-derived organic template for osteogenesis. Biofabrication 2020, 12, 35022. [Google Scholar] [CrossRef] [PubMed]
- Blazewicz, M.; Plenk, H. Carbon materials in the treatment of soft and hard tissue injuries. Eur. Cells Mater. 2001, 2, 21–29. [Google Scholar] [CrossRef]
- Nair, M.; Nancy, D.; Krishnan, A.G.; Anjusree, G.S.; Vadukumpully, S.; Nair, S.V. Graphene oxide nanoflakes incorporated gelatin-hydroxyapatite scaffolds enhance osteogenic differentiation of human mesenchymal stem cells. Nanotechnology 2015, 26. [Google Scholar] [CrossRef]
- Liu, H.; Xi, P.; Xie, G.; Shi, Y.; Hou, F.; Huang, L.; Chen, F.; Zeng, Z.; Shao, C.; Wang, J. Simultaneous reduction and surface functionalization of graphene oxide for hydroxyapatite mineralization. J. Phys. Chem. C 2012, 116, 3334–3341. [Google Scholar] [CrossRef]
- Siqueira, I.A.W.B.; Corat, M.A.F.; Cavalcanti, B.D.N.; Neto, W.A.R.; Martin, A.A.; Bretas, R.E.S.; Marciano, F.R.; Lobo, A.O. In vitro and in vivo studies of novel poly(D,L-lactic acid), superhydrophilic carbon nanotubes, and nanohydroxyapatite scaffolds for bone regeneration. ACS Appl. Mater. Interfaces 2015, 7, 9385–9398. [Google Scholar] [CrossRef]
- Jeong, H.S.; Venkatesan, J.; Kim, S.K. Hydroxyapatite-fucoidan nanocomposites for bone tissue engineering. Int. J. Biol. Macromol. 2013, 57, 138–141. [Google Scholar] [CrossRef]
- Lee, M.; Ku, S.H.; Ryu, J.; Park, C.B. Mussel-inspired functionalization of carbon nanotubes for hydroxyapatite mineralization. J. Mater. Chem. 2010, 20, 8848–8853. [Google Scholar] [CrossRef]
- Yan, P.; Wang, J.; Wang, L.; Liu, B.; Lei, Z.; Yang, S. The in vitro biomineralization and cytocompatibility of polydopamine coated carbon nanotubes. Appl. Surf. Sci. 2011, 257, 4849–4855. [Google Scholar] [CrossRef]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaushik, N.; Nhat Nguyen, L.; Kim, J.H.; Choi, E.H.; Kumar Kaushik, N. Strategies for Using Polydopamine to Induce Biomineralization of Hydroxyapatite on Implant Materials for Bone Tissue Engineering. Int. J. Mol. Sci. 2020, 21, 6544. https://doi.org/10.3390/ijms21186544
Kaushik N, Nhat Nguyen L, Kim JH, Choi EH, Kumar Kaushik N. Strategies for Using Polydopamine to Induce Biomineralization of Hydroxyapatite on Implant Materials for Bone Tissue Engineering. International Journal of Molecular Sciences. 2020; 21(18):6544. https://doi.org/10.3390/ijms21186544
Chicago/Turabian StyleKaushik, Neha, Linh Nhat Nguyen, June Hyun Kim, Eun Ha Choi, and Nagendra Kumar Kaushik. 2020. "Strategies for Using Polydopamine to Induce Biomineralization of Hydroxyapatite on Implant Materials for Bone Tissue Engineering" International Journal of Molecular Sciences 21, no. 18: 6544. https://doi.org/10.3390/ijms21186544
APA StyleKaushik, N., Nhat Nguyen, L., Kim, J. H., Choi, E. H., & Kumar Kaushik, N. (2020). Strategies for Using Polydopamine to Induce Biomineralization of Hydroxyapatite on Implant Materials for Bone Tissue Engineering. International Journal of Molecular Sciences, 21(18), 6544. https://doi.org/10.3390/ijms21186544