Cerebral Vasodilator Property of Poly(ADP-Ribose) Polymerase Inhibitor (PJ34) in the Neonatal and Adult Mouse Is Mediated by the Nitric Oxide Pathway
Abstract
:1. Introduction
2. Results
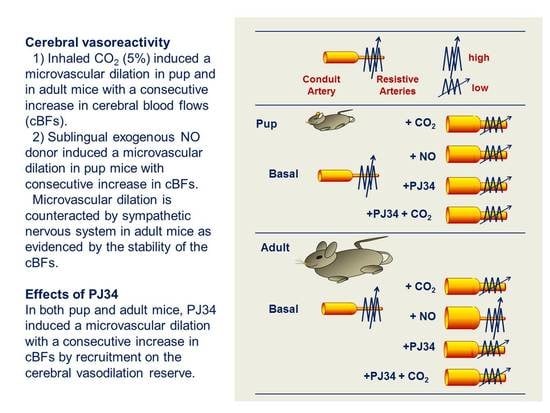
2.1. Cerebrovascular Reactivity (CVR) during Brain Development
2.2. Effect of PJ34 on Cerebrovascular Reactivity
2.3. PJ34 Cerebrovascular Reactivity is Mediated by NOS Activity
2.4. Endothelial NOS Mediates the PJ34 Effects
3. Discussion
- Using NOS inhibitors in the P10 mice, namely L-NMMA (a pan inhibitor) and 7-NI (a more selective nNOS inhibitor), the reduction in BFV appears similar, suggesting that nNOS is important in the neonatal brain for vasodilation compared to the eNOS, and that L-NMMA exerts preferentially its effect through the inhibition of nNOS. Using L-NIO, the reduction in BFV is not so drastic at 1 h after injection, but this inhibitor completely reverses the PJ34 effect.
- In contrast, in the adult mice, L-NMMA mainly blocks the eNOS (at a similar level obtained with L-NIO), and both inhibitors counteract the PJ34 effect.
- Finally, 7-NI reduces BFVs in the P10 brain, in agreement with key role of nNOS in BF regulation [30], which is not the case in the adult brain. However, this inhibitor does not counteract the PJ34 effect in both neonatal and adult brain.
4. Material and Methods
4.1. Ethics Statement
4.2. Animals and Inclusion/Exclusion
4.3. Drug and Study Design
4.4. Ultrasound Imaging
4.5. Cerebrovascular Reactivity (CVR) to Carbon Dioxide (CO2) and/or to Exogenous NO-Donor
4.6. Arterial Blood Pressure and Blood Gases Analysis
4.7. NOS Activity
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Besson, V.C. Drug targets for traumatic brain injury from poly(ADP-ribose)polymerase pathway modulation. Br. J. Pharmacol. 2009, 157, 695–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berger, N.A.; Besson, V.C.; Boulares, A.H.; Bürkle, A.; Chiarugi, A.; Clark, R.S.; Curtin, N.J.; Cuzzocrea, S.; Dawson, T.M.; Dawson, V.L.; et al. Opportunities for the repurposing of PARP inhibitors for the therapy of non-oncological diseases. Br. J. Pharmacol. 2018, 175, 192–222. [Google Scholar] [CrossRef] [PubMed]
- Ducrocq, S.; Benjelloun, N.; Plotkine, M.; Ben-Ari, Y.; Charriaut-Marlangue, C. Poly(ADP-ribose) synthase inhibition reduces ischemic injury and inflammation in neonatal rat brain. J. Neurochem. 2000, 74, 2504–2511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joly, L.M.; Benjelloun, N.; Plotkine, M.; Charriaut-Marlangue, C. Distribution of poly(ADP-ribosyl)ation and cell death after cerebral ischemia in the neonatal rat. Pediatr. Res. 2003, 53, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Hagberg, H.; Wilson, M.A.; Matsushita, H.; Zhu, C.; Lange, M.; Gustavsson, M.; Poitras, M.F.; Dawson, T.M.; Dawson, V.L.; Northington, F.; et al. PARP-1 gene disruption in mice preferentially protects males from perinatal brain injury. J. Neurochem. 2004, 90, 1068–1075. [Google Scholar] [CrossRef]
- Liu, F.; Lang, J.; Li, J.; Benashski, S.E.; Siegel, M.; Xu, Y.; McCullough, L.D. Sex differences in the response to poly(ADP-ribose) polymerase-1 deletion and caspase inhibition after stroke. Stroke 2011, 42, 1090–1096. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Won, S.J.; Xu, Y.; Swanson, R.A. Targeting microglial activation in stroke therapy: Pharmacological tools and gender effects. Curr. Med. Chem. 2014, 21, 2146–2155. [Google Scholar] [CrossRef]
- Charriaut-Marlangue, C.; Leconte, C.; Csaba, Z.; Chafa, L.; Pansiot, J.; Talatizi, M.; Simon, K.; Moretti, R.; Marchand-Leroux, C.; Baud, O.; et al. Sex differences in the effects of PARP inhibition on microglial phenotypes following neonatal stroke. Brain Behav. Immun. 2018, 73, 375–389. [Google Scholar] [CrossRef]
- Soriano, F.G.; Pacher, P.; Mabley, J.; Liaudet, L.; Szabó, C. Rapid reversal of the diabetic endothelial dysfunction by pharmacological inhibition of poly(ADP-ribose) polymerase. Circ. Res. 2001, 89, 684–691. [Google Scholar] [CrossRef] [Green Version]
- Pacher, P.; Mabley, J.G.; Soriano, F.G.; Liaudet, L.; Szabó, C. Activation of poly(ADP-ribose) polymerase contributes to the endothelial dysfunction associated with hypertension and aging. Int. J. Mol. Med. 2002, 9, 659–664. [Google Scholar] [CrossRef]
- Yilmaz, B.; Sahin, P.; Ordueri, E.; Celik-Ozenci, C.; Tasatargil, A. Poly(ADP-ribose) polymerase inhibition improves endothelin-1-induced endothelial dysfunction in rat thoracic aorta. Upsala J. Med. Sci. 2014, 119, 215–222. [Google Scholar] [CrossRef]
- Iadecola, C. The neurovascular unit coming of age: A journey through neurovascular coupling in health and disease. Neuron 2017, 96, 17–42. [Google Scholar] [CrossRef] [Green Version]
- Bonnin, P.; Mazighi, M.; Charriaut-Marlangue, C.; Kubis, N. Early collateral recruitment after stroke in infants and adults. Stroke 2019, 50, 2604–2611. [Google Scholar] [CrossRef]
- Fernandez-Lopez, D.; Faustino, J.; Derugin, N.; Vexler, Z.S. Acute and chronic vascular responses to experimental focal arterial stroke in the neonate rat. Transl. Stroke Res. 2013, 4, 179–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willmot, M.; Gibson, C.; Gray, L.; Murphy, S.; Bath, P. Nitric oxide synthase inhibitors in experimental ischemic stroke and their effects on infarct size and cerebral blood flow: A systematic review. Free Radic. Biol. Med. 2005, 39, 412–425. [Google Scholar] [CrossRef]
- Harb, R.; Whiteus, C.; Freitas, C.; Grutzendler, J. In vivo imaging of cerebral microvascular plasticity from birth to death. J. Cereb. Blood Flow Metab. 2013, 33, 146–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du Bois, M.; Bowman, P.D.; Goldstein, G.W. Angiogenesis in developing rat brain: An in vivo and in vitro study. Brain Res. 1985, 355, 219–223. [Google Scholar]
- Ogunshola, O.O.; Stewart, W.B.; Mihalcik, V.; Solli, T.; Madri, J.A.; Ment, L.R. Neuronal VEGF expression correlates with angiogenesis in postnatal developing rat brain. Dev. Brain Res. 2000, 119, 139–153. [Google Scholar] [CrossRef]
- Iwai, M.; Cao, G.; Yin, W.; Stetler, R.A.; Liu, J.; Chen, J. Erythropoietin promotes neuronal replacement through revascularization and neurogenesis after neonatal hypoxia/ischemia in rats. Stroke 2007, 38, 2795–2803. [Google Scholar] [CrossRef] [Green Version]
- Leger, P.L.; Bonnin, P.; Nguyen, T.; Renolleau, S.; Baud, O.; Charriaut-Marlangue, C. Ischemic postconditioning fails to protect against neonatal cerebral stroke. PLoS ONE 2012, 7, e49695. [Google Scholar] [CrossRef]
- Leger, P.L.; Bonnin, P.; Renolleau, S.; Baud, O.; Charriaut-Marlangue, C. Ischemic postconditioning in cerebral ischemia: Differences between the immature and mature brain? Int. J. Dev. Neurosci. 2015, 45, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Schulz, J.M.; Al-Khazraji, B.K.; Shoemaker, J.K. Sodium nitroglycerin induces middle cerebral artery vasodilatation in young, healthy adults. Exp. Physiol. 2018, 103, 1047–1055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tassorelli, C.; Blandini, F.; Costa, A.; Preza, E.; Nappi, G. Nitroglycerin-induced activation of monoaminergic transmission in the rat. Cephalalgiae 2002, 22, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.X.; Long, J.P. Effects of nitroglycerin on release, synthesis and metabolism of norepinephrine and activation of tyrosine hydroxylase in guinea-pigs. Eur. J. Pharmacol. 1991, 199, 27–33. [Google Scholar] [CrossRef]
- Ma, S.X.; Long, J.P. Positive chronotropic and inotropic responses to release of norepinephrine from sympathetic nerve terminals produced by nitroglycerin in atria. Arch. Int. Pharmacodyn. Ther. 1991, 309, 125–136. [Google Scholar]
- Ma, S.X.; Schmid, P.G., Jr.; Long, J.P. Noradrenergic mechanisms and the cardiovascular actions of nitroglycerin. Life Sci. 1994, 55, 1595–1603. [Google Scholar] [CrossRef]
- Tabsh, K.; Nuwayhid, B.; Murad, S.; Ushioda, E.; Erkkola, R.; Brinkman, C.R., 3rd; Assali, N.S. Circulatory effects of chemical sympathectomy in fetal, neonatal, and adult sheep. Am. J. Physiol. 1982, 243, H113–H122. [Google Scholar] [CrossRef]
- Santizo, R.; Baughman, V.L.; Pelligrino, D.A. Relative contributions from neuronal and endothelial nitric oxide synthases to regional cerebral blood flow changes during forebrain ischemia in rats. Neuroreport 2000, 11, 1549–1553. [Google Scholar] [CrossRef]
- Hagioka, S.; Takeda, Y.; Zhang, S.; Sato, T.; Morita, K. Effects of 7-nitroindazole and N- nitro-l-arginine methyl ester on changes in cerebral blood flow and nitric oxide production preceding development of hyperbaric oxygen-induced seizures in rats. Neurosci. Lett. 2005, 382, 206–210. [Google Scholar] [CrossRef]
- Bonnin, P.; Leger, P.L.; Villapol, S.; Deroide, N.; Gressens, P.; Pocard, M.; Renolleau, S.; Baud, O.; Charriaut-Marlangue, C. Dual action of NO synthases on blood flow and infarct volume consecutive to neonatal focal cerebral ischemia. Exp. Neurol. 2012, 236, 50–57. [Google Scholar] [CrossRef] [Green Version]
- Pacher, P.; Szabó, C. Role of poly(ADP-ribose) polymerase 1 (PARP-1) in cardiovascular diseases: The therapeutic potential of PARP inhibitors. Cardiovasc. Drug Rev. 2007, 25, 235–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keegan, A.; Cotter, M.A.; Cameron, N.E. Effects of diabetes and treatment with the antioxidant alpha-lipoic acid on endothelial and neurogenic responses of corpus cavernosum in rats. Diabetologia 1999, 42, 343–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibson, T.M.; Cotter, M.A.; Cameron, N.E. Effects of poly(ADP-ribose) polymerase inhibition on dysfunction of non-adrenergic non-cholinergic neurotransmission in gastric fundus in diabetic rats. Nitric Oxide 2006, 15, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Li, W.J.; Peng, Y.; Zhou, J.; Li, B.; Wang, H.; Zhang, J.; Wang, Z. Poly(ADP-ribose) polymerase inhibition improves erectile function by activation of nitric oxide/cyclic guanosine monophosphate pathway in diabetic rats. J. Sex. Med. 2012, 9, 1319–1327. [Google Scholar] [CrossRef] [PubMed]
- Wan, Z.H.; Li, W.Z.; Li, Y.Z.; Chen, L.; Li, G.H.; Hu, W.F.; Peng, S.; Yu, J.J.; Gui, F. Poly(ADP-Ribose) polymerase inhibition improves erectile function in diabetic rats. J. Sex. Med. 2011, 8, 1002–1014. [Google Scholar] [CrossRef] [PubMed]
- El Amki, M.; Lerouet, D.; Garraud, M.; Teng, F.; Beray-Berthat, V.; Coqueran, B.; Barsacq, B.; Abbou, C.; Palmier, B.; Marchand-Leroux, C.; et al. Improved reperfusion and vasculoprotection by the Poly(ADP-ribose) polymerase inhibitor PJ34 after stroke and thrombolysis in mice. Mol. Neurobiol. 2018, 55, 9156–9168. [Google Scholar] [CrossRef]
- Bonnin, P.; Pansiot, J.; Paven, E.; Eloi, M.; Renolleau, S.; Baud, O.; Leger, P.L.; Charriaut-Marlangue, C. Controlled arterial reflow after ischemia induces better outcomes in the juvenile rat brain. J. Cereb. Blood Flow Metab. 2017, 37, 3091–3096. [Google Scholar] [CrossRef] [Green Version]
- Vaz, S.; Falkmer, T.; Passmore, A.E.; Parsons, R.; Andreou, P. The case for using the repeatability coefficient when calculating test-retest reliability. PLoS ONE 2013, 8, e73990. [Google Scholar] [CrossRef]
- Cifuentes, D.; Poittevin, M.; Bonnin, P.; Ngkelo, A.; Kubis, N.; Merkulova-Rainon, T.; Levy, B.O. Inactivation of nitric oxide synthesis exacerbates the development of alzheimer disease pathology in APPPS1 mice (Amyloid Precursor Protein/Presenilin-1). Hypertension 2017, 70, 613–623. [Google Scholar] [CrossRef]
- Bush, P.A.; Gonzalez, N.E.; Griscavage, J.M.; Ignarro, L.J. Nitric oxide synthase from cerebellum catalyzes the formation of equimolar quantities of nitric oxide and citrulline from L-arginine. Biochem. Biophys. Res. Commun. 1992, 185, 960–966. [Google Scholar] [CrossRef]
- Manivet, P.; Mouillet-Richard, S.; Callebert, J.; Nebigil, C.G.; Maroteaux, L.; Hosoda, S.; Kellermann, O.; Launay, J.M. PDZ-dependent activation of nitric-oxide synthases by the serotonin 2B receptor. J. Biol. Chem. 2000, 275, 9324–9331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, V.E. Revised standards for statistical evidence. Proc. Natl. Acad Sci. USA 2013, 110, 19313–19317. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Mean BFV (cm/s) | Air | CO2 | p Value (CO2 vs. Air) | Air | NO | p Value (NO vs. Air) | |
|---|---|---|---|---|---|---|---|
| Pup Mice | P10 (n = 7) | 13.3 ± 2.2 | 16.1 ± 2.6 | 0.0016 | 12.2 ± 2.3 | 16.1 ± 3.3 | 0.0007 |
| P12 (n = 7) | 15.8 ± 1.6 | 19.4 ± 1.8 | 0.0011 | 15.6 ± 2.0 | 18.9 ± 3.2 | 0.0034 | |
| p value P12 vs. P10 | 0.0003 | 0.0001 | 0.0003 | 0.0035 | |||
| Adult Mice | P90 (n = 7) | 14.6 ± 1.4 | 16.9 ± 1.5 | <0.0001 | 14.5 ± 1.0 | 13.1 ± 0.8 | 0.0028 |
| p value P90 vs. P12 | NS | NS | 0.0002 | NA | |||
| P365 (n = 6) | 15.4 ± 1.9 | 19.4± 2.0 | <0.0001 | 15.2 ± 1.2 | 13.4 ± 1.7 | 0.0045 | |
| p value P365 vs. P90 | NS | NS | NS | NS |
| Mean BFV (cm/s) | Basal | PJ34 + H1 | p Value (PJ34 vs. Basal) | PJ34 + H48 | p Value (PJ34 vs. Basal) | ||
|---|---|---|---|---|---|---|---|
| Pup Mice | P 10 (n = 7) | Air | 13.3 ± 2.2 | 15.0 ± 0.9 | NS | ||
| CO2 | 16.1 ± 2.6 | 15.3 ± 0.7 | NS | ||||
| p value CO2 vs. air | 0.0016 | NS | |||||
| P 12 (n = 7) | Air | 15.8 ± 1.6 | 19.1 ± 1.9 | 0.0043 | |||
| CO2 | 19.4 ± 1.8 | 20.5 ± 1.4 | NS | ||||
| p value CO2 vs. air | 0.0011 | NS | |||||
| Adult Mice | P 90 (n = 7) | Air | 14.6 ± 1.4 | 15.0 ± 0.8 | NS | 16.1 ± 0.9 | 0.0049 |
| CO2 | 16.9 ± 1.5 | 15.8 ± 0.7 | NS | 16.7 ± 0.5 | NS | ||
| p value CO2 vs. air | <0.0001 | NS | NS | ||||
| P 365 (n = 6) | Air | 15.7± 1.7 | 17.7 ± 1.2 | 0.0033 | 17.5 ± 2.0 | 0.0024 | |
| CO2 | 19.7 ± 1.7 | 19.6 ± 0.7 | NS | 19.6 ± 2.2 | NS | ||
| p value CO2 vs. air | <0.0001 | <0.0001 | 0.0002 | ||||
| Heart Rate (bpm) | Basal | PJ34 + H1 | p Value (PJ34 vs. Basal) | PJ34 + H48 | p Value (PJ34 vs. Basal) | ||
|---|---|---|---|---|---|---|---|
| Pup Mice | P 10 (n = 7) | Air | 431 ± 62 | 526 ± 61 | NS | ||
| CO2 | 340 ± 44 | 413 ± 37 | NS | ||||
| p value CO2 vs. air | 0.0003 | 0.0010 | |||||
| P 12 (n = 7) | Air | 546 ± 61 | 603 ± 27 | NS | |||
| CO2 | 480 ± 39 | 497 ± 32 | NS | ||||
| p value CO2 vs. air | NS | 0.0001 | |||||
| Adult Mice | P 90 (n = 7) | Air | 465 ± 64 | 438 ± 43 | NS | 474 ± 14 | NS |
| CO2 | 443 ± 45 | 450 ± 17 | NS | 489 ± 52 | NS | ||
| p value CO2 vs. air | NS | NS | NS | ||||
| P 365 (n = 6) | Air | 481 ± 65 | 486 ± 39 | NS | 489 ± 12 | NS | |
| CO2 | 496 ± 73 | 515 ± 31 | NS | 496 ± 73 | |||
| p value CO2 vs. air | NS | NS | NS | ||||
| Systolic BP (mmHg) | HR (bpm) | pH | pCO2 (mmHg) | pO2 (mmHg) | SatO2 (%) | |
|---|---|---|---|---|---|---|
| Air | 82 ± 11 | 465 ± 64 | 7.35 ± 0.10 | 29.5 ± 6.1 | 90.7 ± 18.8 | 91.9 ± 10.5 |
| CO2 | 81 ± 10 | 443± 45 | 7.17 ± 0.09 | 46.8 ± 9.3 | 84.2 ± 11.9 | 81.1 ± 13.4 |
| p value (CO2 vs. Air) | NS | NS | 0.0036 | 0.0014 | NS | NS |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonnin, P.; Charriaut-Marlangue, C.; Pansiot, J.; Boutigny, A.; Launay, J.-M.; Besson, V.C. Cerebral Vasodilator Property of Poly(ADP-Ribose) Polymerase Inhibitor (PJ34) in the Neonatal and Adult Mouse Is Mediated by the Nitric Oxide Pathway. Int. J. Mol. Sci. 2020, 21, 6569. https://doi.org/10.3390/ijms21186569
Bonnin P, Charriaut-Marlangue C, Pansiot J, Boutigny A, Launay J-M, Besson VC. Cerebral Vasodilator Property of Poly(ADP-Ribose) Polymerase Inhibitor (PJ34) in the Neonatal and Adult Mouse Is Mediated by the Nitric Oxide Pathway. International Journal of Molecular Sciences. 2020; 21(18):6569. https://doi.org/10.3390/ijms21186569
Chicago/Turabian StyleBonnin, Philippe, Christiane Charriaut-Marlangue, Julien Pansiot, Alexandre Boutigny, Jean-Marie Launay, and Valérie C. Besson. 2020. "Cerebral Vasodilator Property of Poly(ADP-Ribose) Polymerase Inhibitor (PJ34) in the Neonatal and Adult Mouse Is Mediated by the Nitric Oxide Pathway" International Journal of Molecular Sciences 21, no. 18: 6569. https://doi.org/10.3390/ijms21186569
APA StyleBonnin, P., Charriaut-Marlangue, C., Pansiot, J., Boutigny, A., Launay, J. -M., & Besson, V. C. (2020). Cerebral Vasodilator Property of Poly(ADP-Ribose) Polymerase Inhibitor (PJ34) in the Neonatal and Adult Mouse Is Mediated by the Nitric Oxide Pathway. International Journal of Molecular Sciences, 21(18), 6569. https://doi.org/10.3390/ijms21186569