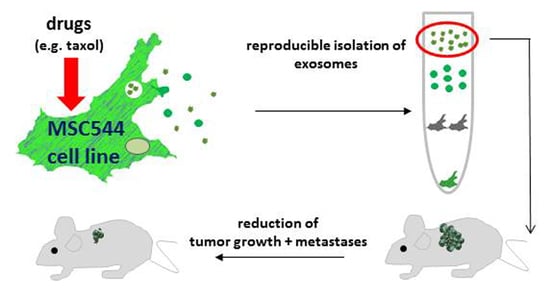
Anti-Tumor Effects of Exosomes Derived from Drug-Incubated Permanently Growing Human MSC
Abstract
:1. Introduction
2. Results
2.1. Characterisation of MSC544-Derived Microvesicles and Exosomes
2.2. In Vitro Anti-Tumor Activity of MSC544-Derived Microvesicles and Exosomes
2.3. In Vivo Application of Taxol-Treated MSC544-Derived Exosomes
2.4. Further Drug Administration to MSC544-Derived Exosomes
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Preparation and Analysis of MSC544-Derived Control Exosomes and Chemotherapeutic-Loaded Exosomes
4.3. Immunoblot Analysis of Exosomes
4.4. In Vitro Cytotoxicity Measurements of Exosomes by Fluoroscan Assay
4.5. In Vivo Experiments
- Treatment 1: Intravenous application (100 µL) of MSC544-derived control exosomes.
- Treatment 2: Oral gavage application (100 µL) of taxol-treated MSC544-derived exosomes. Oral application was performed using plastic feeding tubes (18 ga × 30 mm) (Instech Laboratories, Plymouth, PA, USA).
- Treatment 3: Intravenous application (100 µL) of taxol-treated MSC544-derived exosomes
4.6. Transcript Analysis by RT-PCR
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CAFs | carcinoma-associated fibroblasts |
| EVs | extracellular vesicles |
| hUC | human umbilical cord |
| miR | micro RNA |
| MSC | mesenchymal stroma/stem-like cells |
| NTA | nanoparticle tracking analysis |
| TNBC | triple negative breast cancer |
| TRAIL | tumor necrosis factor-related apoptosis-inducing ligand |
References
- Squillaro, T.; Peluso, G.; Galderisi, U. Clinical trials with mesenchymal stem cells: An update. Cell Transpl. 2016, 25, 829–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phinney, D.G.; Pittenger, M.F. Concise review: MSC-derived exosomes for cell-free therapy. Stem Cells 2017, 35, 851–858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golchin, A.; Farahany, T.Z.; Khojasteh, A.; Soleimanifar, F.; Ardeshirylajimi, A. The clinical trials of mesenchymal stem cell therapy in skin diseases: An update and concise review. Curr. Stem Cell Res. Ther. 2019, 14, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Scuteri, A.; Monfrini, M. Mesenchymal stem cells as new therapeutic approach for diabetes and pancreatic disorders. Int. J. Mol. Sci. 2018, 19, 2783. [Google Scholar] [CrossRef] [Green Version]
- Melzer, C.; Jacobs, R.; Dittmar, T.; Pich, A.; Von Der Ohe, J.; Yang, Y.; Hass, R. Reversible growth-arrest of a spontaneously-derived human MSC-like cell line. Int. J. Mol. Sci. 2020, 21, 4752. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I. Mesenchymal stem cells: Time to change the name. Stem Cells Transl. Med. 2017, 6, 1445–1451. [Google Scholar] [CrossRef] [Green Version]
- Boregowda, S.V.; Booker, C.N.; Phinney, D.G. Mesenchymal stem cells: The moniker fits the science. Stem Cells 2017, 36, 7–10. [Google Scholar] [CrossRef] [Green Version]
- Otte, A.; Bucan, V.; Reimers, K.; Hass, R. Mesenchymal stem cells maintain long-term In Vitro stemness during explant culture. Tissue Eng. Part C Methods 2013, 19, 937–948. [Google Scholar] [CrossRef]
- Hu, C.; Li, L. Preconditioning influences mesenchymal stem cell properties In Vitro and In Vivo. J. Cell. Mol. Med. 2018, 22, 1428–1442. [Google Scholar] [CrossRef] [Green Version]
- Corselli, M.; Chen, C.-W.; Sun, B.; Yap, S.; Rubin, J.P.; Péault, B. The tunica adventitia of human arteries and veins as a source of mesenchymal stem cells. Stem Cells Dev. 2012, 21, 1299–1308. [Google Scholar] [CrossRef] [Green Version]
- Viswanathan, S.; Shi, Y.; Galipeau, J.; Krampera, M.; Leblanc, K.; Martin, I.; Nolta, J.; Phinney, D.; Sensebe, L. Mesenchymal stem versus stromal cells: International Society for Cell & Gene Therapy (ISCT®) Mesenchymal Stromal Cell committee position statement on nomenclature. Cytotherapy 2019, 21, 1019–1024. [Google Scholar] [CrossRef] [PubMed]
- Hass, R.; Kasper, C.; Böhm, S.; Jacobs, R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun. Signal. 2011, 9, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrell, C.R.; Jovicic, N.; Djonov, V.; Arsenijevic, N.; Volarevic, V. Mesenchymal stem cell-derived exosomes and other extracellular vesicles as new remedies in the therapy of inflammatory diseases. Cells 2019, 8, 1605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Freitas, D.; Kim, H.S.; Fabijanic, K.; Li, Z.; Chen, H.; Mark, M.T.; Molina, H.; Benito-Martin, A.; Bojmar, L.; et al. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat. Cell Biol. 2018, 20, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Tkach, M.; Théry, C. Communication by extracellular vesicles: Where we are and where we need to go. Cell 2016, 164, 1226–1232. [Google Scholar] [CrossRef] [Green Version]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nature 2007, 9, 654–659. [Google Scholar] [CrossRef] [Green Version]
- Momen-Heravi, F.; Balaj, L.; Alian, S.; Mantel, P.-Y.; Halleck, A.E.; Trachtenberg, A.J.; Soria, C.E.; Oquin, S.; Bonebreak, C.M.; Saracoglu, E.; et al. Current methods for the isolation of extracellular vesicles. Biol. Chem. 2013, 394, 1253–1262. [Google Scholar] [CrossRef]
- Yang, Y.; Bucan, V.; Baehre, H.; Von Der Ohe, J.; Otte, A.; Hass, R. Acquisition of new tumor cell properties by MSC-derived exosomes. Int. J. Oncol. 2015, 47, 244–252. [Google Scholar] [CrossRef] [Green Version]
- Coffman, L.G.; Choi, Y.J.; McLean, K.; Allen, B.L.; di Magliano, M.P.; Buckanovich, R.J. Human carcinoma-associated mesenchymal stem cells promote ovarian cancer chemotherapy resistance via a BMP4/HH signaling loop. Oncotarget 2016, 7, 6916–6932. [Google Scholar] [CrossRef] [Green Version]
- Mandel, K.; Yang, Y.; Schambach, A.; Glage, S.; Otte, A.; Hass, R. Mesenchymal stem cells directly interact with breast cancer cells and promote tumor cell growth In Vitro and In Vivo. Stem Cells Dev. 2013, 22, 3114–3127. [Google Scholar] [CrossRef]
- Melzer, C.; Von Der Ohe, J.; Hass, R. Concise review: Crosstalk of mesenchymal stroma/stem-like cells with cancer cells provides therapeutic potential. Stem Cells 2018, 36, 951–968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Otte, A.; Hass, R. Human mesenchymal stroma/stem cells exchange membrane proteins and alter functionality during interaction with different tumor cell lines. Stem Cells Dev. 2014, 24, 1205–1222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, R.; Chen, X.; Song, H.; Bie, Q.; Zhang, B. Dual role of MSC-derived exosomes in tumor development. Stem Cells Int. 2020, 2020, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Morton, L.M.; Dores, G.M.; Schonfeld, S.J.; Linet, M.S.; Sigel, B.S.; Lam, C.J.K.; Tucker, M.A.; Curtis, R.E. Association of chemotherapy for solid tumors with development of therapy-related myelodysplastic syndrome or acute myeloid leukemia in the modern era. JAMA Oncol. 2019, 5, 318. [Google Scholar] [CrossRef] [Green Version]
- Chitwood, C.A.; Dietzsch, C.; Jacobs, G.; McArdle, T.; Freeman, B.T.; Banga, A.; Noubissi, F.K.; Ogle, B.M. Breast tumor cell hybrids form spontaneously in vivo and contribute to breast tumor metastases. APL Bioeng. 2018, 2, 031907. [Google Scholar] [CrossRef] [Green Version]
- Melzer, C.; Von Der Ohe, J.; Hass, R. In Vivo cell fusion between mesenchymal stroma/stem-like cells and breast cancer cells. Cancers 2019, 11, 185. [Google Scholar] [CrossRef] [Green Version]
- Melzer, C.; Von Der Ohe, J.; Hass, R. MSC stimulate ovarian tumor growth during intercellular communication but reduce tumorigenicity after fusion with ovarian cancer cells. Cell Commun. Signal. 2018, 16, 67. [Google Scholar] [CrossRef] [Green Version]
- Melzer, C.; Rehn, V.; Yang, Y.; Baehre, H.; Von Der Ohe, J.; Hass, R. Taxol-loaded MSC-derived exosomes provide a therapeutic vehicle to target metastatic breast cancer and other carcinoma cells. Cancers 2019, 11, 798. [Google Scholar] [CrossRef] [Green Version]
- Martins, T.; Catita, J.A.M.; Rosa, I.M.; Silva, O.A.B.d.C.E.; Henriques, A.G. Exosome isolation from distinct biofluids using precipitation and column-based approaches. PLoS ONE 2018, 13, e0198820. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, A.K.; Aqil, F.; Jeyabalan, J.; Spencer, W.A.; Beck, J.; Gachuki, B.W.; AlHakeem, S.S.; Oben, K.; Munagala, R.; Bondada, S.; et al. Milk-derived exosomes for oral delivery of paclitaxel. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1627–1636. [Google Scholar] [CrossRef]
- Tomayko, M.M.; Reynolds, C.P. Determination of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother. Pharm. 1989, 24, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Otte, A.; Rauprich, F.; Von Der Ohe, J.; Yang, Y.; Kommoss, F.; Feuerhake, F.; Hillemanns, P.; Hass, R. c-Met inhibitors attenuate tumor growth of small cell hypercalcemic ovarian carcinoma (SCCOHT) populations. Oncotarget 2015, 6, 31640–31658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furlani, D.; Ugurlucan, M.; Ong, L.; Bieback, K.; Pittermann, E.; Westien, I.; Wang, W.; Yerebakan, C.; Li, W.; Gaebel, R.; et al. Is the intravascular administration of mesenchymal stem cells safe? Mesenchymal stem cells and intravital microscopy. Microvasc. Res. 2009, 77, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.-O.; Han, J.W.; Kim, J.-M.; Cho, H.-J.; Park, C.; Lee, N.; Kim, N.-W.; Yoon, Y.-S. Malignant tumor formation after transplantation of short-term cultured bone marrow mesenchymal stem cells in experimental myocardial infarction and diabetic neuropathy. Circ. Res. 2011, 108, 1340–1347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, M.G.; Tung, L.; Sekar, R.B.; Chang, C.Y.; Cysyk, J.; Dong, P.; Marbán, E.; Abraham, M.R. Proarrhythmic potential of mesenchymal stem cell transplantation revealed in an In Vitro coculture model. Circulation 2006, 113, 1832–1841. [Google Scholar] [CrossRef]
- Lou, G.; Chen, Z.; Zheng, M.; Liu, Y. Mesenchymal stem cell-derived exosomes as a new therapeutic strategy for liver diseases. Exp. Mol. Med. 2017, 49, e346. [Google Scholar] [CrossRef]
- Yin, K.; Wang, S.; Zhao, R.C. Exosomes from mesenchymal stem/stromal cells: A new therapeutic paradigm. Biomark. Res. 2019, 7, 8. [Google Scholar] [CrossRef] [Green Version]
- Vakhshiteh, F.; Atyabi, F.; Ostad, S.N. Mesenchymal stem cell exosomes: A two-edged sword in cancer therapy. Int. J. Nanomed. 2019, 14, 2847–2859. [Google Scholar] [CrossRef] [Green Version]
- Whiteside, T.L. Exosome and mesenchymal stem cell cross-talk in the tumor microenvironment. Semin. Immunol. 2017, 35, 69–79. [Google Scholar] [CrossRef]
- Dittmer, J.; Lange, T.; Leyh, B.; Dittmer, J. Protein and growth-modulatory effects of carcinoma-associated fibroblasts on breast cancer cells: Role of interleukin-6. Int. J. Oncol. 2019, 56, 258–272. [Google Scholar] [CrossRef] [Green Version]
- Melzer, C.; Yang, Y.; Hass, R. Interaction of MSC with tumor cells. Cell Commun. Signal. 2016, 14, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farías, V.D.A.; O’Valle, F.; Alonso-Lerma, B.; De Almodóvar, C.R.; Lopez-Peñalver, J.J.; Nieto, A.; Santos, A.; Fernandez-Gil, B.I.; Guerra-Librero, A.; Ruiz-Ruiz, C.; et al. Human mesenchymal stem cells enhance the systemic effects of radiotherapy. Oncotarget 2015, 6, 31164–31180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farías, V.D.A.; O’Valle, F.; Serrano-Saenz, S.; Anderson, P.O.; Andrés-León, E.; Lopez-Peñalver, J.J.; Tovar-Martín, I.; Nieto, A.; Santos, A.; Martin, F.; et al. Exosomes derived from mesenchymal stem cells enhance radiotherapy-induced cell death in tumor and metastatic tumor foci. Mol. Cancer 2018, 17, 122. [Google Scholar] [CrossRef]
- Feng, H.; Zhao, J.-K.; Schiergens, T.S.; Wang, P.-X.; Ou, B.-C.; Al-Sayegh, R.; Li, M.-L.; Lu, A.-G.; Yin, S.; E Thasler, W. Bone marrow-derived mesenchymal stromal cells promote colorectal cancer cell death under low-dose irradiation. Br. J. Cancer 2018, 118, 353–365. [Google Scholar] [CrossRef] [Green Version]
- McClain-Caldwell, I.; Vitale-Cross, L.A.; Mayer, B.; Krepuska, M.; Boyajian, M.; Myneni, V.; Martin, D.; Marko, K.; Nemeth, K.; Mezey, É.; et al. Immunogenic potential of human bone marrow mesenchymal stromal cells is enhanced by hyperthermia. Cytotherapy 2018, 20, 1437–1444. [Google Scholar] [CrossRef] [PubMed]
- Farias, V.D.A.; Tovar, I.; Del Moral, R.; O’Valle, F.; Expósito, J.; Oliver, F.J.; De Almodóvar, J.M.R. Enhancing the bystander and abscopal effects to improve radiotherapy outcomes. Front. Oncol. 2020, 9, 9. [Google Scholar] [CrossRef] [Green Version]
- Martín, I.T.; Guerrero, R.; Lopez-Peñalver, J.J.; Expósito, J.; De Almodóvar, J.M.R. Rationale for the use of radiation-activated mesenchymal stromal/stem cells in acute respiratory distress syndrome. Cells 2020, 9, 2015. [Google Scholar] [CrossRef]
- Lee, R.H.; Yoon, N.; Reneau, J.C.; Prockop, D.J. Preactivation of human MSCs with TNF-α enhances tumor-suppressive activity. Cell Stem Cell 2012, 11, 825–835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shamili, F.H.; Bayegi, H.R.; Salmasi, Z.; Sadri, K.; Mahmoudi, M.; Kalantari, M.; Ramezani, M.; Abnous, K. Exosomes derived from TRAIL-engineered mesenchymal stem cells with effective anti-tumor activity in a mouse melanoma model. Int. J. Pharm. 2018, 549, 218–229. [Google Scholar] [CrossRef]
- Zhang, B.; Shen, L.; Shi, H.; Pan, Z.; Wu, L.; Yan, Y.; Zhang, X.; Mao, F.; Qian, H.; Xu, W. Exosomes from human umbilical cord mesenchymal stem cells: Identification, purification, and biological characteristics. Stem Cells Int. 2016, 2016, 1–11. [Google Scholar] [CrossRef]
- Ding, Y.; Cao, F.; Sun, H.; Wang, Y.; Liu, S.; Wu, Y.; Cui, Q.; Mei, W.; Li, F. Exosomes derived from human umbilical cord mesenchymal stromal cells deliver exogenous miR-145-5p to inhibit pancreatic ductal adenocarcinoma progression. Cancer Lett. 2019, 442, 351–361. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Li, W.; Zheng, T.; Liu, D.; Zhao, S. Human umbilical cord mesenchymal stem cells-derived exosomes deliver microRNA-375 to downregulate ENAH and thus retard esophageal squamous cell carcinoma progression. J. Exp. Clin. Cancer Res. 2020, 39, 140. [Google Scholar] [CrossRef]
- Yeung, C.L.A.; Co, N.-N.; Tsuruga, T.; Yeung, T.-L.; Kwan, S.Y.; Leung, C.S.; Li, Y.; Lu, E.S.; Kwan, K.; Wong, K.-K.; et al. Exosomal transfer of stroma-derived miR21 confers paclitaxel resistance in ovarian cancer cells through targeting APAF1. Nat. Commun. 2016, 7, 11150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hass, R. Retrodifferentiation and cell death. Crit. Rev. Oncog. 1994, 5, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Hass, R. Rejuvenation in distinct cell populations—What does it mean? Exp. Gerontol. 2009, 44, 634–638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meinhardt, G.; Roth, J.; Hass, R. Activation of protein kinase C relays pdistinct signaling pathways in the same cell type: Differentiation and caspase-mediated apoptosis. Cell Death Differ. 2000, 7, 795–803. [Google Scholar] [CrossRef] [Green Version]
- Morishita, M.; Takahashi, Y.; Nishikawa, M.; Takakura, Y. Pharmacokinetics of exosomes—An important factor for elucidating the biological roles of exosomes and for the development of exosome-based therapeutics. J. Pharm. Sci. 2017, 106, 2265–2269. [Google Scholar] [CrossRef] [Green Version]
- Kalimuthu, S.; Gangadaran, P.; Rajendran, R.L.; Zhu, L.; Oh, J.M.; Lee, H.W.; Gopal, A.; Baek, S.H.; Jeong, S.Y.; Lee, S.-W.; et al. A new approach for loading anticancer drugs into mesenchymal stem cell-derived exosome mimetics for cancer therapy. Front. Pharmacol. 2018, 9, 9. [Google Scholar] [CrossRef]
- Mendt, M.; Rezvani, K.; Shpall, E.J. Mesenchymal stem cell-derived exosomes for clinical use. Bone Marrow Transplant. 2019, 54, 789–792. [Google Scholar] [CrossRef]
- Melzer, C.; Von Der Ohe, J.; Lehnert, H.; Ungefroren, H.; Hass, R. Cancer stem cell niche models and contribution by mesenchymal stroma/stem cells. Mol. Cancer 2017, 16, 28. [Google Scholar] [CrossRef] [Green Version]
- Melzer, C.; von der Ohe, J.; Otterbein, H.; Ungefroren, H.; Hass, R. Changes in uPA, PAI-1, and TGF-beta production during breast cancer cell interaction with human mesenchymal stroma/stem-like cells (MSC). Int. J. Mol. Sci. 2019, 20, 2630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melzer, C.; Von Der Ohe, J.; Hass, R. Enhanced metastatic capacity of breast cancer cells after interaction and hybrid formation with mesenchymal stroma/stem cells (MSC). Cell Commun. Signal. 2018, 16, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otte, A.; Rauprich, F.; Hillemanns, P.; Park-Simon, T.-J.; Von Der Ohe, J.; Hass, R. In Vitro and in vivotherapeutic approach for a small cell carcinoma of the ovary hypercalcaemic type using a SCCOHT-1 cellular model. Orphanet J. Rare Dis. 2014, 9, 126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Théry, C.; Amigorena, S.; Raposo, G.; Clayton, A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol. 2006, 30, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [Green Version]
- Melzer, C.; Von Der Ohe, J.; Hass, R. In Vitro fusion of normal and neoplastic breast epithelial cells with human mesenchymal stroma/stem cells partially involves tumor necrosis factor receptor signaling. Stem Cells 2018, 36, 977–989. [Google Scholar] [CrossRef] [Green Version]






| Vesicles | Zeta Potential (mV) | Concentration (Vesicles/mL) |
|---|---|---|
| Control exosomes | −30.3 | 8.8 × 1011 |
| Taxol microvesicles | −19.1 | 2.0 × 1011 |
| Taxol exosomes | −15.1 | 2.8 × 1011 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melzer, C.; Ohe, J.v.d.; Hass, R. Anti-Tumor Effects of Exosomes Derived from Drug-Incubated Permanently Growing Human MSC. Int. J. Mol. Sci. 2020, 21, 7311. https://doi.org/10.3390/ijms21197311
Melzer C, Ohe Jvd, Hass R. Anti-Tumor Effects of Exosomes Derived from Drug-Incubated Permanently Growing Human MSC. International Journal of Molecular Sciences. 2020; 21(19):7311. https://doi.org/10.3390/ijms21197311
Chicago/Turabian StyleMelzer, Catharina, Juliane von der Ohe, and Ralf Hass. 2020. "Anti-Tumor Effects of Exosomes Derived from Drug-Incubated Permanently Growing Human MSC" International Journal of Molecular Sciences 21, no. 19: 7311. https://doi.org/10.3390/ijms21197311
APA StyleMelzer, C., Ohe, J. v. d., & Hass, R. (2020). Anti-Tumor Effects of Exosomes Derived from Drug-Incubated Permanently Growing Human MSC. International Journal of Molecular Sciences, 21(19), 7311. https://doi.org/10.3390/ijms21197311