Uncovering the Pharmacological Mechanism of Stemazole in the Treatment of Neurodegenerative Diseases Based on a Network Pharmacology Approach
Abstract
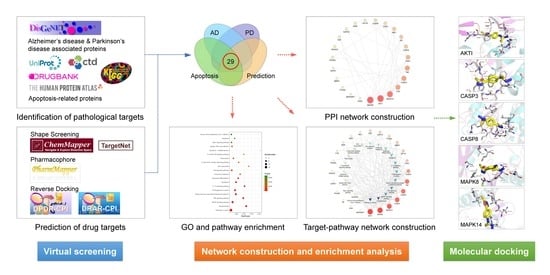
:1. Introduction
2. Results
2.1. Screening of Potential Targets
2.2. Protein–Protein Interaction (PPI) Network Construction and Analysis
2.3. GO and KEGG Pathway Enrichment Analyses
2.4. Molecular Docking
3. Discussion
4. Materials and Methods
4.1. Identification of Pathological Targets
4.2. Virtual Screening of Drug Targets
4.3. PPI Network Construction and Analysis
4.4. GO and KEGG Pathway Enrichment Analyses
4.5. Molecular Docking
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| GO | gene ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| AKT1 | RAC-alpha serine/threonine-protein kinase |
| CASP3 | caspase-3 |
| CASP8 | caspase-8 |
| MAPK8 | mitogen-activated protein kinase 8 |
| MAPK14 | mitogen-activated protein kinase 14 |
| AD | Alzheimer’s disease |
| PD | Parkinson’s disease |
| ST | Stemazole |
| Aβ | beta-amyloid |
| MPTP | 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine |
| ABL1 | Tyrosine-protein kinase ABL1 |
| ACHE | Acetylcholinesterase |
| AR | Androgen receptor |
| CASP9 | Caspase-9 |
| CDK5 | Cyclin-dependent-like kinase 5 |
| CSF1R | Macrophage colony-stimulating factor 1 receptor |
| DAPK1 | Death-associated protein kinase 1 |
| DRD3 | D(3) dopamine receptor |
| ESR1 | Estrogen receptor |
| FGF2 | Fibroblast growth factor 2 |
| FYN | Tyrosine-protein kinase Fyn |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| HGF | Hepatocyte growth factor |
| HMGCR | 3-hydroxy-3-methylglutaryl-coenzyme A reductase |
| IGF1R | Insulin-like growth factor 1 receptor |
| INSR | Insulin receptor |
| MAPK8IP1 | C-Jun-amino-terminal kinase-interacting protein 1 |
| MMP9 | Matrix metalloproteinase-9 |
| PIK3CG | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform |
| PPARD | Peroxisome proliferator-activated receptor delta |
| PPARG | Peroxisome proliferator-activated receptor gamma |
| PPIF | Peptidyl-prolyl cis-trans isomerase F, mitochondrial |
| PTGS2 | Prostaglandin G/H synthase 2 |
| TNF | Tumor necrosis factor |
| PPI | Protein–protein interaction |
| DHF | 7,8-dihydroxyflavone |
References
- Friedlander, R.M. Mechanisms of disease: Apoptosis and caspases in neurodegenerative diseases. N. Engl. J. Med. 2003, 348, 1365–1375. [Google Scholar] [CrossRef] [PubMed]
- Wyss-Coray, T. Ageing, neurodegeneration and brain rejuvenation. Nature 2016, 539, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Reitz, C.; Brayne, C.; Mayeux, R. Epidemiology of Alzheimer disease. Nat. Rev. Neurol. 2011, 7, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef]
- Patterson, C. The State of the Art of Dementia Research: New Frontiers; World Alzheimer Report 2018; Alzheimer’s Disease International (ADI): London, UK, 2018. [Google Scholar]
- Dorsey, E.R.; Bloem, B.R. The Parkinson Pandemic-A Call to Action. JAMA Neurol. 2018, 75, 9–10. [Google Scholar] [CrossRef]
- Bachurin, S.O.; Bovina, E.V.; Ustyugov, A.A. Drugs in Clinical Trials for Alzheimer’s Disease: The Major Trends. Med. Res. Rev. 2017, 37, 1186–1225. [Google Scholar] [CrossRef]
- Fernandez, H.H. 2015 Update on Parkinson disease. Clevel. Clin. J. Med. 2015, 82, 563–568. [Google Scholar] [CrossRef]
- Cummings, J.; Aisen, P.S.; DuBois, B.; Frölich, L.; Jack, C.R.; Jones, R.W.; Morris, J.C.; Raskin, J.; Dowsett, S.A.; Scheltens, P. Drug development in Alzheimer’s disease: The path to 2025. Alzheimer’s Res. Ther. 2016, 8, 39. [Google Scholar] [CrossRef] [Green Version]
- Charvin, D.; Medori, R.; Hauser, R.A.; Rascol, O. Therapeutic strategies for Parkinson disease: Beyond dopaminergic drugs. Nat. Rev. Drug Discov. 2018, 17, 804–822. [Google Scholar] [CrossRef]
- Palop, J.J.; Chin, J.; Mucke, L. A network dysfunction perspective on neurodegenerative diseases. Nature 2006, 443, 768–773. [Google Scholar] [CrossRef]
- Lev, N.; Melamed, E.; Offen, D. Apoptosis and Parkinson’s disease. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2003, 27, 245–250. [Google Scholar] [CrossRef]
- Singh, S.; Srivastava, A.; Srivastava, P.; Dhuriya, Y.K.; Pandey, A.; Kumar, D.; Rajpurohit, C.S. Advances in Stem Cell Research—A Ray of Hope in Better Diagnosis and Prognosis in Neurodegenerative Diseases. Front. Mol. Biosci. 2016, 3, 72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoddard-Bennett, T.; Pera, R.R. Treatment of Parkinson’s Disease through Personalized Medicine and Induced Pluripotent Stem Cells. Cells 2019, 8, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yue, C.; Jing, N. The promise of stem cells in the therapy of Alzheimer’s disease. Transl. Neurodegener. 2015, 4, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, F.; Baremberg, D.; Gao, J.; Duan, J.; Lu, X.; Zhang, N.; Chen, Q. Development of stem cell-based therapy for Parkinson’s disease. Transl. Neurodegener. 2015, 4, 16. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Wang, W.; Sun, Y.; Han, M. Synthesis and biological evaluation of a novel human stem/progenitor cells proliferation activator: 4-(4-(5-mercapto-1,3,4-oxadiazol-2-yl)phenyl) thiosemicarbazide (Stemazole). Eur. J. Med. Chem. 2011, 46, 2930–2936. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, X.; Li, H.; Xu, S.; Zhang, X.; Liu, Y.; Han, M.; Wen, J. Stemazole promotes survival and preserves stemness in human embryonic stem cells. FEBS J. 2018, 285, 531–541. [Google Scholar] [CrossRef]
- Han, M.; Liu, Y.; Tan, Q.; Zhang, B.; Wang, W.; Liu, J.; Zhang, X.-J.; Wang, Y.-Y.; Zhang, J.-M. Therapeutic efficacy of stemazole in a beta-amyloid injection rat model of Alzheimer’s disease. Eur. J. Pharmacol. 2011, 657, 104–110. [Google Scholar] [CrossRef]
- Guo, Z.; Xu, S.; Du, N.; Liu, J.; Huang, Y.; Han, M. Neuroprotective effects of stemazole in the MPTP-induced acute model of Parkinson’s disease: Involvement of the dopamine system. Neurosci. Lett. 2016, 616, 152–159. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Tan, Q.; Zhang, Y.; Zhang, J.; Zhao, C.; Lu, S.; Qiao, J.; Han, M. Pharmacokinetics and absolute oral bioavailability of stemazole by UPLC-MS/MS and its bio-distribution through tritium labeling. Drug Test. Anal. 2019. [Google Scholar] [CrossRef]
- Hopkins, A.L. Network pharmacology: The next paradigm in drug discovery. Nat. Chem. Biol. 2008, 4, 682–690. [Google Scholar] [CrossRef]
- Park, M.; Park, S.-Y.; Lee, H.-J.; Kim, C.-E. A Systems-Level Analysis of Mechanisms of Platycodon grandiflorum Based on A Network Pharmacological Approach. Molecules 2018, 23, 2841. [Google Scholar] [CrossRef] [Green Version]
- Boezio, B.; Audouze, K.; Ducrot, P.; Taboureau, O. Network-based Approaches in Pharmacology. Mol. Inform. 2017, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pieper, A.A.; Xie, S.; Capota, E.; Estill, S.J.; Zhong, J.; Long, J.M.; Becker, G.L.; Huntington, P.; Goldman, S.E.; Shen, C.-H.; et al. Discovery of a Proneurogenic, Neuroprotective Chemical. Cell 2010, 142, 39–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voorhees, J.R.; Remy, M.T.; Cintron-Perez, C.J.; El Rassi, E.; Khan, M.Z.; Dutca, L.M.; Yin, T.C.; McDaniel, L.N.; Williams, N.S.; Brat, D.J.; et al. (-)-P7C3-S243 Protects a Rat Model of Alzheimer’s Disease From Neuropsychiatric Deficits and Neurodegeneration Without Altering Amyloid Deposition or Reactive Glia. Biol. Psychiatry 2018, 84, 488–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Jesus-Cortes, H.; Xu, P.; Drawbridge, J.; Estill, S.J.; Huntington, P.; Tran, S.; Britt, J.; Tesla, R.; Morlock, L.; Naidoo, J.; et al. Neuroprotective efficacy of aminopropyl carbazoles in a mouse model of Parkinson disease. Proc. Natl. Acad. Sci. USA 2012, 109, 17010–17015. [Google Scholar] [CrossRef] [Green Version]
- Yin, T.C.; Britt, J.K.; De Jesus-Cortes, H.; Lu, Y.; Genova, R.M.; Khan, M.Z.; Voorhees, J.R.; Shao, J.; Katzman, A.C.; Huntington, P.J.; et al. P7C3 Neuroprotective Chemicals Block Axonal Degeneration and Preserve Function after Traumatic Brain Injury. Cell Rep. 2014, 8, 1731–1740. [Google Scholar] [CrossRef] [Green Version]
- Jang, S.-W.; Liu, X.; Yepes, M.; Shepherd, K.R.; Miller, G.W.; Liu, Y.; Wilson, W.D.; Xiao, G.; Blanchi, B.; Sun, Y.E.; et al. A selective TrkB agonist with potent neurotrophic activities by 7,8-dihydroxyflavone. Proc. Natl. Acad. Sci. USA 2010, 107, 2687–2692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Liu, X.; Schroeder, J.P.; Chan, C.-B.; Song, M.; Yu, S.P.; Weinshenker, D.; Ye, K. 7,8-Dihydroxyflavone Prevents Synaptic Loss and Memory Deficits in a Mouse Model of Alzheimer’s Disease. Neuropsychopharmacology 2014, 39, 638–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, M.; Peng, Q.; Liu, X.; Jin, J.; Hou, Z.; Zhang, J.; Mori, S.; Ross, C.A.; Ye, K.; Duan, W. Small-molecule TrkB receptor agonists improve motor function and extend survival in a mouse model of Huntingtons disease. Hum. Mol. Genet. 2013, 22, 2462–2470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korkmaz, O.T.; Aytan, N.; Carreras, I.; Choi, J.-K.; Kowall, N.W.; Jenkins, B.G.; Dedeoglu, A. 7,8-Dihydroxyflavone improves motor performance and enhances lower motor neuronal survival in a mouse model of amyotrophic lateral sclerosis. Neurosci. Lett. 2014, 566, 286–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, E.K.; Choi, E.-J. Pathological roles of MAPK signaling pathways in human diseases. Biochim. Biophys. Acta Mol. Basis Dis. 2010, 1802, 396–405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griffin, R.J.; Moloney, A.; Kelliher, M.; Johnston, J.A.; Ravid, R.; Dockery, P.; O’Connor, R.; O’Neill, C. Activation of Akt/PKB, increased phosphorylation of Akt substrates and loss and altered distribution of Akt and PTEN are features of Alzheimer’s disease pathology. J. Neurochem. 2005, 93, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.; Nidadavolu, P.; Durgadoss, L.; Ravindranath, V. Critical cysteines in Akt1 regulate its activity and proteasomal degradation: Implications for neurodegenerative diseases. Free Radic. Biol. Med. 2014, 74, 118–128. [Google Scholar] [CrossRef]
- Coffey, E.T.; Smiciene, G.; Hongisto, V.; Cao, J.; Brecht, S.; Herdegen, T.; Courtney, M.J. c-Jun N-terminal protein kinase (JNK) 2/3 is specifically activated by stress, mediating c-Jun activation, in the presence of constitutive JNK1 activity in cerebellar neurons. J. Neurosci. 2002, 22, 4335–4345. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, M.; Du, Y.; Zhang, W.; Bai, M.; Zhang, Z.; Li, Z.; Miao, J. Inhibition of c-Jun N-Terminal Kinase Activation Reverses Alzheimer Disease Phenotypes in APPswe/PS1dE9 Mice. Ann. Neurol. 2015, 77, 637–654. [Google Scholar] [CrossRef]
- Mehan, S.; Meena, H.; Sharma, D.; Sankhla, R. JNK: A Stress-Activated Protein Kinase Therapeutic Strategies and Involvement in Alzheimer’s and Various Neurodegenerative Abnormalities. J. Mol. Neurosci. 2011, 43, 376–390. [Google Scholar] [CrossRef]
- Giraldo, E.; Lloret, A.; Fuchsberger, T.; Viña, J. Aβ and tau toxicities in Alzheimer’s are linked via oxidative stress-induced p38 activation: Protective role of vitamin E. Redox Biol. 2014, 2, 873–877. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Cheng, J.; North, B.J.; Wei, W. Functional analyses of major cancer-related signaling pathways in Alzheimer’s disease etiology. Biochim. Biophys. Acta. Rev. Cancer 2017, 1868, 341–358. [Google Scholar] [CrossRef]
- Corti, O.; Hampe, C.; Koutnikova, H.; Darios, F.; Jacquier, S.; Prigent, A.; Robinson, J.C.; Pradier, L.; Ruberg, M.; Mirande, M.; et al. The p38 subunit of the aminoacyl-tRNA synthetase complex is a Parkin substrate: Linking protein biosynthesis and neurodegeneration. Hum. Mol. Genet. 2003, 12, 1427–1437. [Google Scholar] [CrossRef] [Green Version]
- Louneva, N.; Cohen, J.W.; Han, L.Y.; Talbot, K.; Wilson, R.S.; Bennett, D.A.; Trojanowski, J.Q.; Arnold, S.E. Caspase-3 is enriched in postsynaptic densities and increased in Alzheimer’s disease. Am. J. Pathol. 2008, 173, 1488–1495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivins, K.J.; Thornton, P.L.; Rohn, T.T.; Cotman, C.W. Neuronal apoptosis induced by beta-amyloid is mediated by caspase-8. Neurobiol. Dis. 1999, 6, 440–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Amelio, M.; Cavallucci, V.; Middei, S.; Marchetti, C.; Pacioni, S.; Ferri, A.; Diamantini, A.; De Zio, D.; Carrara, P.; Battistini, L.; et al. Caspase-3 triggers early synaptic dysfunction in a mouse model of Alzheimer’s disease. Nat. Neurosci. 2011, 14, 69–76. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.S.; Ha, J.Y.; Yang, S.J.; Son, J.H. A Novel Non-Apoptotic Role of Procaspase-3 in the Regulation of Mitochondrial Biogenesis Activators. J. Cell. Biochem. 2018, 119, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Pinero, J.; Bravo, A.; Queralt-Rosinach, N.; Gutierrez-Sacristan, A.; Deu-Pons, J.; Centeno, E.; Garcia-Garcia, J.; Sanz, F.; Furlong, L.I. DisGeNET: A comprehensive platform integrating information on human disease-associated genes and variants. Nucleic Acids Res. 2017, 45, D833–D839. [Google Scholar] [CrossRef]
- Uhlen, M.; Oksvold, P.; Fagerberg, L.; Lundberg, E.; Jonasson, K.; Forsberg, M.; Zwahlen, M.; Kampf, C.; Wester, K.; Hober, S.; et al. Towards a knowledge-based Human Protein Atlas. Nat. Biotechnol. 2010, 28, 1248–1250. [Google Scholar] [CrossRef]
- Wishart, D.S.; Feunang, Y.D.; Guo, A.C.; Lo, E.J.; Marcu, A.; Grant, J.R.; Sajed, T.; Johnson, D.; Li, C.; Sayeeda, Z.; et al. DrugBank 5.0: A major update to the DrugBank database for 2018. Nucleic Acids Res. 2018, 46, D1074–D1082. [Google Scholar] [CrossRef]
- Bateman, A.; Martin, M.-J.; Orchard, S.; Magrane, M.; Alpi, E.; Bely, B.; Bingley, M.; Britto, R.; Bursteinas, B.; Busiello, G.; et al. UniProt: A worldwide hub of protein knowledge. Nucleic Acids Res. 2019, 47, D506–D515. [Google Scholar] [CrossRef] [Green Version]
- Davis, A.P.; Grondin, C.J.; Johnson, R.J.; Sciaky, D.; McMorran, R.; Wiegers, J.; Wiegers, T.C.; Mattingly, C.J. The Comparative Toxicogenomics Database: Update 2019. Nucleic Acids Res. 2019, 47, D948–D954. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09 Revision A.1; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- ACD/Labs. ACD/ChemSketch, Version 2018.2; Advanced Chemistry Development, Inc.: Toronto, ON, Canada, 2019. [Google Scholar]
- Huang, H.; Zhang, G.; Zhou, Y.; Lin, C.; Chen, S.; Lin, Y.; Mai, S.; Huang, Z. Reverse Screening Methods to Search for the Protein Targets of Chemopreventive Compounds. Front. Chem. 2018, 6, 138. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shen, Y.; Wang, S.; Li, S.; Zhang, W.; Liu, X.; Lai, L.; Pei, J.; Li, H. PharmMapper 2017 update: A web server for potential drug target identification with a comprehensive target pharmacophore database. Nucleic Acids Res. 2017, 45, W356–W360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, H.; Chen, J.; Shi, L.; Mikailov, M.; Zhu, H.; Wang, K.; He, L.; Yang, L. DRAR-CPI: A server for identifying drug repositioning potential and adverse drug reactions via the chemical-protein interactome. Nucleic Acids Res. 2011, 39, W492–W498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, H.; Zhang, P.; Cao, X.H.; Du, D.; Ye, H.; Huang, H.; Li, C.; Qin, S.; Wan, C.; Shi, L.; et al. DPDR-CPI, a server that predicts Drug Positioning and Drug Repositioning via Chemical-Protein Interactome. Sci. Rep. 2016, 6, 35996. [Google Scholar] [CrossRef] [Green Version]
- Yao, Z.-J.; Dong, J.; Che, Y.-J.; Zhu, M.-F.; Wen, M.; Wang, N.-N.; Wang, S.; Lu, A.-P.; Cao, D.-S. TargetNet: A web service for predicting potential drug-target interaction profiling via multi-target SAR models. J. Comput. Aided Mol. Des. 2016, 30, 413–424. [Google Scholar] [CrossRef]
- Gong, J.; Cai, C.; Liu, X.; Ku, X.; Jiang, H.; Gao, D.; Li, H. ChemMapper: A versatile web server for exploring pharmacology and chemical structure association based on molecular 3D similarity method. Bioinformatics 2013, 29, 1827–1829. [Google Scholar] [CrossRef] [Green Version]
- Szklarczyk, D.; Franceschini, A.; Wyder, S.; Forslund, K.; Heller, D.; Huerta-Cepas, J.; Simonovic, M.; Roth, A.; Santos, A.; Tsafou, K.P.; et al. STRING v10: Protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015, 43, D447–D452. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Assenov, Y.; Ramirez, F.; Schelhorn, S.-E.; Lengauer, T.; Albrecht, M. Computing topological parameters of biological networks. Bioinformatics 2008, 24, 282–284. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.; Li, M.; Wang, J.; Pan, Y.; Wu, F.-X. CytoNCA: A cytoscape plugin for centrality analysis and evaluation of protein interaction networks. Biosystems 2015, 127, 67–72. [Google Scholar] [CrossRef]
- Raudvere, U.; Kolberg, L.; Kuzmin, I.; Arak, T.; Adler, P.; Peterson, H.; Vilo, J. g:Profiler: A web server for functional enrichment analysis and conversions of gene lists (2019 update). Nucleic Acids Res. 2019, 47, W191–W198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burley, S.K.; Berman, H.M.; Bhikadiya, C.; Bi, C.; Chen, L.; Di Costanzo, L.; Christie, C.; Dalenberg, K.; Duarte, J.M.; Dutta, S.; et al. RCSB Protein Data Bank: Biological macromolecular structures enabling research and education in fundamental biology, biomedicine, biotechnology and energy. Nucleic Acids Res. 2018, 47, D464–D474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| No. | Symbol | Protein Name | Database |
|---|---|---|---|
| 1 | ABL1 | Tyrosine-protein kinase ABL1 | DRAR-CPI, DPDR-CPI, PharmMapper |
| 2 | ACHE | Acetylcholinesterase | ChemMapper |
| 3 | AKT1 | RAC-alpha serine/threonine-protein kinase | ChemMapper |
| 4 | AR | Androgen receptor | DPDR-CPI, ChemMapper |
| 5 | CASP3 | Caspase-3 | PharmMapper |
| 6 | CASP8 | Caspase-8 | DPDR-CPI |
| 7 | CASP9 | Caspase-9 | TargetNet |
| 8 | CDK5 | Cyclin-dependent-like kinase 5 | ChemMapper |
| 9 | CSF1R | Macrophage colony-stimulating factor 1 receptor | DPDR-CPI |
| 10 | DAPK1 | Death-associated protein kinase 1 | PharmMapper |
| 11 | DRD3 | D(3) dopamine receptor | ChemMapper |
| 12 | ESR1 | Estrogen receptor | DRAR-CPI, PharmMapper, ChemMapper, TargetNet |
| 13 | FGF2 | Fibroblast growth factor 2 | ChemMapper |
| 14 | FYN | Tyrosine-protein kinase Fyn | DPDR-CPI |
| 15 | GAPDH | Glyceraldehyde-3-phosphate dehydrogenase | ChemMapper |
| 16 | HGF | Hepatocyte growth factor | ChemMapper |
| 17 | HMGCR | 3-hydroxy-3-methylglutaryl-coenzyme A reductase | PharmMapper |
| 18 | IGF1R | Insulin-like growth factor 1 receptor | DRAR-CPI |
| 19 | INSR | Insulin receptor | PharmMapper |
| 20 | MAPK14 | Mitogen-activated protein kinase 14 | PharmMapper, ChemMapper |
| 21 | MAPK8 | Mitogen-activated protein kinase 8 | DPDR-CPI, PharmMapper, ChemMapper |
| 22 | MAPK8IP1 | C-Jun-amino-terminal kinase-interacting protein 1 | ChemMapper |
| 23 | MMP9 | Matrix metalloproteinase-9 | DPDR-CPI |
| 24 | PIK3CG | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform | DPDR-CPI, TargetNet |
| 25 | PPARD | Peroxisome proliferator-activated receptor delta | DRAR-CPI |
| 26 | PPARG | Peroxisome proliferator-activated receptor gamma | DRAR-CPI, ChemMapper |
| 27 | PPIF | Peptidyl-prolyl cis-trans isomerase F, mitochondrial | ChemMapper |
| 28 | PTGS2 | Prostaglandin G/H synthase 2 | ChemMapper, TargetNet |
| 29 | TNF | Tumor necrosis factor | DRAR-CPI |
| Degree | Subgragh | Betweenness | Closeness | |
|---|---|---|---|---|
| MAPK8 | 9 | 35.681553 | 164.86667 | 0.6111111 |
| AKT1 | 9 | 33.13798 | 71.3 | 0.5945946 |
| MAPK14 | 8 | 40.777042 | 100.933334 | 0.52380955 |
| FYN | 6 | 23.114271 | 116.72222 | 0.5 |
| CASP3 | 6 | 20.888252 | 63.6 | 0.5 |
| ESR1 | 5 | 16.392994 | 46.566666 | 0.5 |
| AR | 4 | 15.240643 | 53.433334 | 0.47826087 |
| CASP8 | 4 | 9.148983 | 11.6 | 0.4680851 |
| ABL1 | 4 | 14.081305 | 18 | 0.41509435 |
| TNF | 6 | 13.089347 | 8.866667 | 0.4489796 |
| CASP9 | 3 | 7.837092 | 3.4444444 | 0.43137255 |
| CDK5 | 3 | 5.526705 | 8.5 | 0.37931034 |
| INSR | 2 | 3.5084944 | 42 | 0.4 |
| IGF1R | 2 | 2.9850514 | 0 | 0.4 |
| PPARG | 2 | 2.9773748 | 0 | 0.3859649 |
| HGF | 2 | 3.2480063 | 8 | 0.36666667 |
| GAPDH | 2 | 4.990251 | 5.8333335 | 0.36065573 |
| PIK3CG | 2 | 5.3232713 | 2.3333333 | 0.33846155 |
| PTGS2 | 1 | 1.9571668 | 0 | 0.3859649 |
| DAPK1 | 1 | 2.1390505 | 0 | 0.33846155 |
| MMP9 | 1 | 1.957167 | 0 | 0.33846155 |
| FGF2 | 1 | 1.6149884 | 0 | 0.33846155 |
| MAPK8IP1 | 1 | 2.5664337 | 0 | 0.28947368 |
| Receptors | Binding Energy (ΔG)/kcal·moL−1 | Inhibit Constant (Ki)/μM |
|---|---|---|
| CASP3 | −7.45 | 3.45 |
| MAPK14 | −7.21 | 5.17 |
| AKT1 | −7.04 | 6.86 |
| CASP8 | −6.48 | 17.92 |
| MAPK8 | −6.38 | 20.9 |
| Targets | PDB ID | Grid Center | Npts | Spacing | ||
|---|---|---|---|---|---|---|
| AKT1 | 6HHF | 5.230 | 2.277 | 20.620 | 60 60 60 | 0.375 |
| CASP3 | 5IAG | 8.173 | −18.986 | −21.032 | 60 60 60 | 0.375 |
| CASP8 | 3KJN | −5.713 | 17.721 | 16.597 | 60 60 60 | 0.375 |
| MAPK8 | 3O2M | 17.929 | 107.605 | 53.797 | 60 60 60 | 0.375 |
| MAPK14 | 5ETC | 5.881 | 76.862 | 22.002 | 60 60 60 | 0.375 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Li, H.; Zhang, Y.; Zhao, C.; Zhu, Y.; Han, M. Uncovering the Pharmacological Mechanism of Stemazole in the Treatment of Neurodegenerative Diseases Based on a Network Pharmacology Approach. Int. J. Mol. Sci. 2020, 21, 427. https://doi.org/10.3390/ijms21020427
Zhang J, Li H, Zhang Y, Zhao C, Zhu Y, Han M. Uncovering the Pharmacological Mechanism of Stemazole in the Treatment of Neurodegenerative Diseases Based on a Network Pharmacology Approach. International Journal of Molecular Sciences. 2020; 21(2):427. https://doi.org/10.3390/ijms21020427
Chicago/Turabian StyleZhang, Jing, Huajun Li, Yubo Zhang, Chaoran Zhao, Yizi Zhu, and Mei Han. 2020. "Uncovering the Pharmacological Mechanism of Stemazole in the Treatment of Neurodegenerative Diseases Based on a Network Pharmacology Approach" International Journal of Molecular Sciences 21, no. 2: 427. https://doi.org/10.3390/ijms21020427
APA StyleZhang, J., Li, H., Zhang, Y., Zhao, C., Zhu, Y., & Han, M. (2020). Uncovering the Pharmacological Mechanism of Stemazole in the Treatment of Neurodegenerative Diseases Based on a Network Pharmacology Approach. International Journal of Molecular Sciences, 21(2), 427. https://doi.org/10.3390/ijms21020427