GRK2-Mediated Crosstalk Between β-Adrenergic and Angiotensin II Receptors Enhances Adrenocortical Aldosterone Production In Vitro and In Vivo
Abstract
:1. Introduction
2. Results
2.1. Catecholamines and AngII Synergistically Stimulate Aldosterone Secretion from H295R Cells
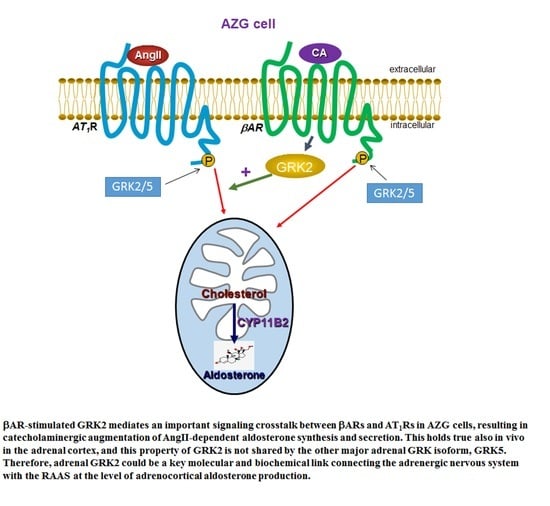
2.2. GRK2, but Not GRK5, Is Essential for the Synergism between Catecholamines and AngII to Stimulate Aldosterone Production
2.3. GRK2, but not GRK5, Is Essential for the Synergistic Aldosterone Synthesis Induction By Catecholamines and AngII In AZG Cells
2.4. βAR-Activated GRK2, but Not GRK5, Is Essential for the Catecholamine-Dependent Enhancement of Adrenal Aldosterone Production In Vivo
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. H295R Cell Culture and Transfections
4.3. Aldosterone Measurements
4.4. Real-Time PCR
4.5. Experimental Animals and Gene Delivery Procedure
4.6. Western Blotting
4.7. Statistical Analyses
Author Contributions
Funding
Conflicts of Interest
References
- Lymperopoulos, A.; Aukszi, B. Angiotensin receptor blocker drugs and inhibition of adrenal beta-arrestin-1-dependent aldosterone production: Implications for heart failure therapy. World J. Cardiol. 2017, 9, 200–206. [Google Scholar] [CrossRef]
- Sztechman, D.; Czarzasta, K.; Cudnoch-Jedrzejewska, A.; Szcaepanska-Sadowska, E.; Zera, T. Aldosterone and mineralocorticoid receptors in regulation of the cardiovascular system and pathological remodelling of the heart and arteries. J. Physiol. Pharmacol. 2018, 69, 829–845. [Google Scholar]
- Parker, B.M.; Wertz, S.L.; Pollard, C.M.; Desimine, V.L.; Maning, J.; McCRrink, K.A.; Lymperopoulos, A. Novel Insights into the Crosstalk between Mineralocorticoid Receptor and G Protein-Coupled Receptors in Heart Adverse Remodeling and Disease. Int. J. Mol. Sci. 2018, 19, 3764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karnik, S.S.; Unal, H.; Kemp, J.R.; Tirupula, K.C.; Eguchi, S.; Wanderheyden, P.M.L.; Thomas, W.G. International Union of Basic and Clinical Pharmacology. XCIX. Angiotensin Receptors: Interpreters of Pathophysiological Angiotensinergic Stimuli [corrected]. Pharmacol. Rev. 2015, 67, 754–819. [Google Scholar] [CrossRef] [Green Version]
- Connell, J.M.; MacKenzie, S.M.; Freel, E.M.; Fraser, R.; Davies, E. A lifetime of aldosterone excess: Long-term consequences of altered regulation of aldosterone production for cardiovascular function. Endocr. Rev. 2008, 29, 133–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tóth, A.D.; Turu, G.; Hunyady, L.; Balla, A. Novel mechanisms of G-protein-coupled receptors functions: AT1 angiotensin receptor acts as a signaling hub and focal point of receptor cross-talk. Best Pract. Res. Clin. Endocrinol. Metab. 2018, 32, 69–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lymperopoulos, A.; Rengo, G.; Zincarelli, C.; Kim, J.; Soltys, S.; Koch, W.J. An adrenal beta-arrestin 1-mediated signaling pathway underlies angiotensin II-induced aldosterone production in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2009, 106, 5825–5830. [Google Scholar] [CrossRef] [Green Version]
- Rainey, W.E.; Saner, K.; Schimmer, B.P. Adrenocortical cell lines. Mol. Cell. Endocrinol. 2004, 228, 23–38. [Google Scholar] [CrossRef]
- Miller, W.L. Steroid hormone synthesis in mitochondria. Mol. Cell. Endocrinol. 2013, 379, 62–73. [Google Scholar] [CrossRef]
- Lymperopoulos, A.; Rengo, G.; Koch, W.J. Adrenergic nervous system in heart failure: Pathophysiology and therapy. Circ. Res. 2013, 113, 739–753. [Google Scholar] [CrossRef]
- Vizi, E.S.; Tóth, I.E.; Szalay, K.S.; Windisch, K.; Orso, E.; Szabo, D.; Vinson, G.P. Catecholamines released from local adrenergic axon terminals are possibly involved in fine tuning of steroid secretion from zona glomerulosa cells: Functional and morphological evidence. J. Endocrinol. 1992, 135, 551–561. [Google Scholar] [CrossRef]
- Szalay, K.S.; Orsó, E.; Jurányi, Z.; Vinson, G.P.; Vizi, E.S. Local non-synaptic modulation of aldosterone production by catecholamines and ATP in rat: Implications for a direct neuronal fine tuning. Horm. Metab. Res. 1998, 30, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T. Mechanism of salt-sensitive hypertension: Focus on adrenal and sympathetic nervous systems. J. Am. Soc. Nephrol. 2014, 25, 1148–1155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kosti, O.; King, P.J.; Hinson, J.P. Tumour-derived human adrenocortical cells express beta-adrenergic receptors: Steroidogenic effects of beta-adrenergic input. Endocr. Res. 2002, 28, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Salazar, N.C.; Vallejos, X.; Siryk, A.; Gengo, G.; Cannavo, A.; Liccardo, D.; De Lucia, C.; Gao, E.; Leosco, D.; Koch, W.J.; et al. GRK2 blockade with βARKct is essential for cardiac β2-adrenergic receptor signaling towards increased contractility. Cell. Commun. Signal. 2013, 11, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lymperopoulos, A. Arrestins in the Cardiovascular System: An Update. Prog. Mol. Biol. Transl. Sci. 2018, 159, 27–57. [Google Scholar]
- Bathgate-Siryk, A.; Dabul, S.; Pandya, K.; Waiklett, K.; Rengo, G.; Cannavo, A.; De Lucia, C.; Liccardo, D.; Gao, E.; Leosco, D.; et al. Negative impact of β-arrestin-1 on post-myocardial infarction heart failure via cardiac and adrenal-dependent neurohormonal mechanisms. Hypertension 2014, 63, 404–412. [Google Scholar] [CrossRef] [Green Version]
- Lymperopoulos, A.; Rengo, G.; Koch, W.J. Adrenal adrenoceptors in heart failure: Fine-tuning cardiac stimulation. Trends. Mol. Med. 2007, 13, 503–511. [Google Scholar] [CrossRef]
- Lymperopoulos, A.; Rengo, G.; Funakoshi, H.; Eckhart, A.D.; Koch, W.J. Adrenal GRK2 upregulation mediates sympathetic overdrive in heart failure. Nat. Med. 2007, 13, 315–323. [Google Scholar] [CrossRef]
- Peterson, Y.K.; Luttrell, L.M. The Diverse Roles of Arrestin Scaffolds in G Protein-Coupled Receptor Signaling. Pharmacol. Rev. 2017, 69, 256–297. [Google Scholar] [CrossRef] [Green Version]
- Yu, Q.; Gratzke, C.; Wang, Y.; Herlemann, A.; Strittmatter, F.; Rutz, B.; Stief, C.G.; Hennenberg, M. Inhibition of prostatic smooth muscle contraction by the inhibitor of G protein-coupled receptor kinase 2/3, CMPD101. Eur. J. Pharmacol. 2018, 831, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Lymperopoulos, A.; Rengo, G.; Zincarelli, C.; Soltys, S.; Koch, W.J. Modulation of adrenal catecholamine secretion by in vivo gene transfer and manipulation of G protein-coupled receptor kinase-2 activity. Mol. Ther. 2008, 16, 302–307. [Google Scholar] [CrossRef] [PubMed]
- de Gasparo, M.; Catt, K.J.; Inagami, T.; Wright, J.W.; Unger, T. International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol. Rev. 2000, 52, 415–472. [Google Scholar] [PubMed]
- Brown, N.J. Contribution of aldosterone to cardiovascular and renal inflammation and fibrosis. Nat. Rev. Nephrol. 2013, 9, 459–469. [Google Scholar] [CrossRef]
- Lymperopoulos, A.; Rengo, G.; Gao, E.; Ebert, S.N.; Dorn, G.W., 2nd; Koch, W.J. Reduction of sympathetic activity via adrenal-targeted GRK2 gene deletion attenuates heart failure progression and improves cardiac function after myocardial infarction. J. Biol. Chem. 2010, 285, 16378–16386. [Google Scholar] [CrossRef] [Green Version]
- Jafferjee, M.; Reyes Valero, T.; Marrero, C.; McCrink, K.A.; Brill, A.; Lymperopoulos, A. GRK2 Up-Regulation Creates a Positive Feedback Loop for Catecholamine Production in Chromaffin Cells. Mol. Endocrinol. 2016, 30, 372–381. [Google Scholar] [CrossRef] [Green Version]
- Lymperopoulos, A.; Rengo, G.; Zincarelli, C.; Kim, J.; Koch, W.J. Adrenal beta-arrestin 1 inhibition in vivo attenuates post-myocardial infarction progression to heart failure and adverse remodeling via reduction of circulating aldosterone levels. J. Am. Coll. Cardiol. 2011, 57, 356–365. [Google Scholar] [CrossRef] [Green Version]
- Gurevich, E.V.; Tesmer, J.J.; Mushegian, A.; Gurevich, V.V. G protein-coupled receptor kinases: More than just kinases and not only for GPCRs. Pharmacol. Ther. 2012, 133, 40–69. [Google Scholar] [CrossRef] [Green Version]
- Tóth, A.D.; Gyombolai, P.; Szalai, B.; Várnai, P.; Turu, G.; Hunyady, L. Angiotensin type 1A receptor regulates β-arrestin binding of the β2-adrenergic receptor via heterodimerization. Mol. Cell. Endocrinol. 2017, 442, 113–124. [Google Scholar] [CrossRef] [Green Version]
- Maniero, C.; Zhou, J.; Shaikh, L.H.; Azizan, E.A.; McFarlane, I.; Neogi, S.; Scudieri, P.; Galietta, L.J.; Brown, M.J. Role of ANO4 in regulation of aldosterone secretion in the zona glomerulosa of the human adrenal gland. Lancet 2015, 385 (Suppl. 1), S62. [Google Scholar] [CrossRef]
- Maniero, C.; Scudieri, P.; Haris Shaikh, L.; Zhao, W.; Gurnell, M.; Galietta, L.J.V.; Brown, M.J. ANO4 (Anoctamin 4) Is a Novel Marker of Zona Glomerulosa That Regulates Stimulated Aldosterone Secretion. Hypertension 2019, 74, 1152–1159. [Google Scholar] [CrossRef] [PubMed]
- Valero, T.R.; Sturchler, E.; Jafferjee, M.; Rengo, G.; Magafa, V.; Cordopatis, P.; McDonald, P.; Koch, W.J.; Lymperopoulos, A. Structure-activity relationship study of angiotensin II analogs in terms of β-arrestin-dependent signaling to aldosterone production. Pharmacol. Res. Perspect. 2016, 4, e00226. [Google Scholar] [CrossRef] [PubMed]
- McCrink, K.A.; Maning, J.; Vu, A.; Jafferjee, M.; Marrero, C.; Brill, A.; Bathgate-Siryk, A.; Dabul, S.; Kouch, W.J.; Lymperopoulos, A. β-Arrestin2 Improves Post-Myocardial Infarction Heart Failure via Sarco(endo)plasmic Reticulum Ca2+-ATPase-Dependent Positive Inotropy in Cardiomyocytes. Hypertension 2017, 70, 972–981. [Google Scholar] [CrossRef] [PubMed]
- Dabul, S.; Bathgate-Siryk, A.; Valero, T.R.; Jafferjee, M.; Sturchler, E.; McDonald, P.; Koch, W.J.; Lymperopoulos, A. Suppression of adrenal βarrestin1-dependent aldosterone production by ARBs: Head-to-head comparison. Sci. Rep. 2015, 5, 8116. [Google Scholar] [CrossRef] [Green Version]
- Pollard, C.M.; Desimine, V.L.; Wertz, S.L.; Perez, A.; Parker, B.M.; Maning, J.; McCrink, K.A.; Shehadeh, L.A.; Lymperopoulos, A. Deletion of Osteopontin Enhances β2-Adrenergic Receptor-Dependent Anti-Fibrotic Signaling in Cardiomyocytes. Int. J. Mol. Sci. 2019, 20, 1396. [Google Scholar] [CrossRef] [Green Version]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pollard, C.M.; Ghandour, J.; Cora, N.; Perez, A.; Parker, B.M.; Desimine, V.L.; Wertz, S.L.; Pereyra, J.M.; Ferraino, K.E.; Patel, J.J.; et al. GRK2-Mediated Crosstalk Between β-Adrenergic and Angiotensin II Receptors Enhances Adrenocortical Aldosterone Production In Vitro and In Vivo. Int. J. Mol. Sci. 2020, 21, 574. https://doi.org/10.3390/ijms21020574
Pollard CM, Ghandour J, Cora N, Perez A, Parker BM, Desimine VL, Wertz SL, Pereyra JM, Ferraino KE, Patel JJ, et al. GRK2-Mediated Crosstalk Between β-Adrenergic and Angiotensin II Receptors Enhances Adrenocortical Aldosterone Production In Vitro and In Vivo. International Journal of Molecular Sciences. 2020; 21(2):574. https://doi.org/10.3390/ijms21020574
Chicago/Turabian StylePollard, Celina M., Jennifer Ghandour, Natalie Cora, Arianna Perez, Barbara M. Parker, Victoria L. Desimine, Shelby L. Wertz, Janelle M. Pereyra, Krysten E. Ferraino, Jainika J. Patel, and et al. 2020. "GRK2-Mediated Crosstalk Between β-Adrenergic and Angiotensin II Receptors Enhances Adrenocortical Aldosterone Production In Vitro and In Vivo" International Journal of Molecular Sciences 21, no. 2: 574. https://doi.org/10.3390/ijms21020574
APA StylePollard, C. M., Ghandour, J., Cora, N., Perez, A., Parker, B. M., Desimine, V. L., Wertz, S. L., Pereyra, J. M., Ferraino, K. E., Patel, J. J., & Lymperopoulos, A. (2020). GRK2-Mediated Crosstalk Between β-Adrenergic and Angiotensin II Receptors Enhances Adrenocortical Aldosterone Production In Vitro and In Vivo. International Journal of Molecular Sciences, 21(2), 574. https://doi.org/10.3390/ijms21020574