Mild Hyperthermia Aggravates Glucose Metabolic Consequences in Repetitive Concussion
Abstract
:1. Introduction
2. Results
2.1. Physiology
2.2. Qualitative Metabolic Consequences
2.3. Quantitative Findings
3. Discussion
4. Methods
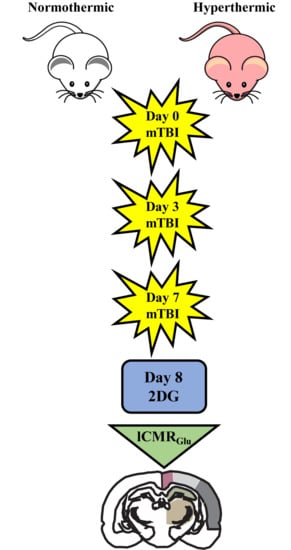
4.1. Weight Drop Injury
4.2. 14C-2-Deoxy-d-Glucose (2DG) Surgical Methods
4.3. Image Processing
4.4. Region of Interest Analysis and Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| TBI | Traumatic brain injury |
| 2DG | 14C-2-deoxy-d-glucose |
| mTBI | Mild traumatic brain injury |
| lCMRGlc | Local cerebral metabolic rate of glucose |
| CBF | Cerebral blood flow |
| CNS | Central nervous system |
| ICU | Intensive care unit |
| ROI | Region of interest |
| Cg | Cingulate cortex |
| M1 | Primary motor cortex |
| M2 | Secondary motor cortex |
| RS | Retrosplenial cortex |
| PtA | Parietal association cortex |
| VM | Visual cortex |
| CPu | Caudate putamen |
| STR | Striatum |
| CA | Cornu ammonis/hippocampus |
| COR | Cortical strip |
| GLUT | Glucose transporter-3 |
| FDG-PET | [18F]fluorodeoxyglucose-positron emission tomography |
| RCTs | Randomized controlled trials |
References
- Taylor, C.A.; Bell, J.M.; Breiding, M.J.; Xu, L. Traumatic Brain Injury-Related Emergency Department Visits, Hospitalizations, and Deaths-United States, 2007 and 2013. MMWR Surveill. Summ. 2017, 66, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Faul, M.; Xu, L.; Wald, M.M.; Coronado, V.G. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths 2002–2006; Centers for Disease Control and Prevention, National Center for Injury Prevention and Control: Atlanta, GA, USA, 2010.
- Coronado, V.G.; Thurman, D.J.; Greenspan, A.I.; Weissman, B.M. Epidemiology. In Neurotrauma and Critical Care of the Brain; Jallo, J., Loftus, C.M., Eds.; Thieme: New York, NY, USA, 2009. [Google Scholar]
- Giza, C.C.; Difiori, J.P. Pathophysiology of sports-related concussion: An update on basic science and translational research. Sports Health 2011, 3, 46–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mittenberg, W.; Canyock, E.M.; Condit, D.; Patton, C. Treatment of post-concussion syndrome following mild head injury. J. Clin. Exp. Neuropsychol. 2001, 23, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Willer, B.; Leddy, J.J. Management of concussion and post-concussion syndrome. Curr. Treat. Options Neurol. 2006, 8, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Giza, C.C.; Hovda, D.A. The Neurometabolic Cascade of Concussion. J. Athl. Train. 2001, 36, 228–235. [Google Scholar] [CrossRef] [Green Version]
- Hoge, C.W.; McGurk, D.; Thomas, J.L.; Cox, A.L.; Engel, C.C.; Castro, C.A. Mild Traumatic Brain Injury in U.S. Soldiers Returning from Iraq. N. Engl. J. Med. 2008, 358, 453–463. [Google Scholar] [CrossRef]
- Yoshino, A.; Hovda, D.A.; Kawamata, T.; Katayama, Y.; Becker, D.P. Dynamic changes in local cerebral glucose utilization following cerebral concussion in rats: Evidence of a hyper-and subsequent hypometabolic state. Brain Res. 1991, 61, 106–119. [Google Scholar] [CrossRef]
- Prins, M.L.; Alexander, D.; Giza, C.C.; Hovda, D.A. Repeated Mild Traumatic Brain Injury: Mechanisms of Cerebral Vulnerability. J. Neurotrauma 2013, 30, 30–38. [Google Scholar] [CrossRef]
- Bergsneider, M.; Hovda, D.A.; McArthur, D.L.; Etchepare, M.; Huang, S.C.; Sehati, N.; Becker, D.P. Metabolic Recovery Following Human Traumatic Brain Injury Based on FDG-PET: Time Course and Relationship to Neurological Disability. J. Head Trauma Rehabil. 2001, 16, 135–148. [Google Scholar] [CrossRef]
- Guskiewicz, K.M.; McCrea, M.; Marshall, S.W.; Cantu, R.C.; Randolph, C.; Barr, W.; Kelly, J.P. Cumulative Effects Associated With Recurrent Concussion in Collegiate Football Players: The NCAA Concussion Study. JAMA 2003, 290, 2549–2555. [Google Scholar] [CrossRef] [Green Version]
- The CDC, NIH, DoD, VA Leadership Panel. Report to Congress on Traumatic Brain Injury in the United States: Understanding the Public Health Problem among Current and Former Military Personnel. 2013. Available online: https://www.cdc.gov/traumaticbraininjury/pubs/congress_military.html (accessed on 11 June 2019).
- Coronado, V.G.; Haileyesus, T.; Cheng, T.A.; Bell, J.M.; Haarbauer-Krupa, J.; Lionbarger, M.R.; Flores-Herrera, J.; McGuire, L.C.; Gilchrist, J. Trends in Sports- and Recreation-Related Traumatic Brain Injuries Treated in US Emergency Departments: The National Electronic Injury Surveillance System-All Injury Program (NEISS-AIP) 2001–2012. J. Head Trauma Rehabil. 2015, 30, 185. [Google Scholar] [CrossRef] [PubMed]
- Greco, T.; Ferguson, L.; Giza, C.; Prins, M.L. Mechanisms underlying vulnerabilities after repeat mild traumatic brain injuries. Exp. Neurol. 2019, 317, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Fehily, B.; Fitzgerald, M. Repeated Mild Traumatic Brain Injury: Potential Mechanisms of Damage. Cell Transplant. 2017, 26, 1131–1155. [Google Scholar] [CrossRef] [PubMed]
- Longhi, L.; Perego, C.; Ortolano, F.; Aresi, S.; Fumagalli, S.; Zanier, E.R.; Stocchetti, N.; De Simoni, M.G. Tumor necrosis factor in traumatic brain injury: Effects of genetic deletion of p55 or p75 receptor. J. Cereb. Blood Flow Metab. 2013, 33, 1182–1189. [Google Scholar] [CrossRef] [Green Version]
- Weil, Z.M.; Gaier, K.R.; Karelina, K. Injury timing alters metabolic, inflammatory and functional outcomes following repeated mild traumatic brain injury. Neurobiol. Dis. 2014, 70, 108–116. [Google Scholar] [CrossRef]
- Hovda, D.A. The neurophysiology of concussion. Prog. Neruol. Surg. 2014, 28, 28–37. [Google Scholar]
- Thompson, H.J.; Kirkness, C.J.; Mitchell, P.H. Intensive care unit management of fever following traumatic brain injury. Intensive Crit. Care Nurs. 2007, 23, 91–96. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.Y.; Gao, G.Y.; Li, W.P.; Yu, M.K.; Zhu, C. Early indicators of prognosis in 846 cases of severe traumatic brain injury. J. Neurotrauma 2002, 19, 869–874. [Google Scholar] [CrossRef]
- Stocchetti, N.; Rossi, S.; Zanier, E.R.; Colombo, A.; Beretta, L.; Citerio, G. Pyrexia in head-injured patients admitted to intensive care. Intensive Care Med. 2002, 28, 1555–1562. [Google Scholar] [CrossRef]
- Truettner, J.S.; Bramlett, H.M.; Dietrich, W.D. Hyperthermia and Mild Traumatic Brain Injury: Effects on Inflammation and the Cerebral Vasculature. J. Neurotrauma 2017, 35, 940–952. [Google Scholar] [CrossRef]
- Titus, D.J.; Furones, C.; Atkins, C.M.; Dietrich, W.D. Emergence of cognitive deficits after mild traumatic brain injury due to hyperthermia. Exp. Neurol. 2015, 263, 254–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakurai, A.; Atkins, C.M.; Alonso, O.F.; Bramlett, H.M.; Dietrich, W.D. Mild Hyperthermia Worsens the Neuropathological Damage Associated with Mild Traumatic Brain Injury in Rats. J. Neurotrauma 2012, 29, 313–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, T.; Bramlett, H.M.; Ruenes, G.; Dietrich, W.D. The effects of early post-traumatic hyperthermia in female and ovariectomized rats. J. Neurotrauma 2004, 21, 842–853. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, W.D.; Alonso, O.; Halley, M.; Busto, R. Delayed posttraumatic brain hyperthermia worsens outcome after fluid percussion brain injury: A light and electron microscopic study in rats. Neurosurgery 1996, 38, 533–541. [Google Scholar] [PubMed]
- Dietrich, W.D.; Bramlett, H.M. Hyperthermia and central nervous system injury. Prog. Brain Res. 2007, 162, 201–217. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, L.; Kogure, K.; Busto, R. Effects of Hypothermia and Hyperthermia on Brain Energy Metabolism. Acta Anaesthesiol. Scand. 1975, 19, 199–205. [Google Scholar] [CrossRef]
- McCulloch, J.; Savaki, H.E.; Jehle, J.; Sokoloff, L. Local Cerebral Glucose Utilization in Hypothermic and Hyperthermic Rats. J. Neurochem. 1982, 39, 255–258. [Google Scholar] [CrossRef]
- Chen, T.; Qian, Y.Z.; Di, X.; Rice, A.; Zhu, J.P.; Bullock, R. Lactate/glucose dynamics after rat fluid percussion brain injury. J. Neurotrauma 2000, 17, 135–142. [Google Scholar] [CrossRef]
- Wapnir, R.A.; Lifshitz, F. Fasting-induced hypoglycemia in experimentally malnourished rats. J. Nutr. 1977, 107, 383–390. [Google Scholar] [CrossRef] [Green Version]
- Loepke, A.W.; McCann, J.C.; Kurth, C.D.; McAuliffe, J.J. The physiologic effects of isoflurane anesthesia in neonatal mice. Anesth. Analg. 2006, 102, 75–80. [Google Scholar] [CrossRef]
- Lee, D.H.; Chung, M.Y.; Lee, J.U.; Kang, D.G.; Paek, Y.W. Changes of glucose transporters in the cerebral adaptation to hypoglycemia. Diabetes Res. Clin. Pract. 2000, 47, 15–23. [Google Scholar] [CrossRef]
- Mannino, C.; Glenn, T.C.; Hovda, D.A.; Vespa, P.M.; McArthur, D.L.; Van Horn, J.D.; Wright, M.J. Acute glucose and lactate metabolism are associated with cognitive recovery following traumatic brain injury. J. Neurosci. Res. 2018, 96, 696–701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Panach, J.; Lull, N.; Lull, J.J.; Ferri, J.; Martinez, C.; Sopena, P.; Robles, M.; Chirivella, J.; Noe, E. A voxel-based analysis of FDG-PET in traumatic brain injury: Regional metabolism and relationship between the thalamus and cortical areas. J. Neurotrauma 2011, 28, 1707–1717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mickley, G.A.; Cobb, B.L.; Farrell, S.T. Brain hyperthermia alters local cerebral glucose utilization: A comparison of hyperthermic agents. Int. J. Hyperth. 1997, 13, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Kimball, A.L.; McCue, P.M.; Petrie, M.A.; Shields, R.K. Whole body heat exposure modulates acute glucose metabolism. Int. J. Hyperth. 2018, 35, 644–651. [Google Scholar] [CrossRef] [Green Version]
- Nunneley, S.A.; Martin, C.C.; Slauson, J.W.; Hearon, C.M.; Nickerson, L.D.; Mason, P.A. Changes in regional cerebral metabolism during systemic hyperthermia in human. J. Appl. Physiol. 1985, 92, 846–851. [Google Scholar] [CrossRef] [Green Version]
- Polderman, K.H. Induced hypothermia and fever control for prevention and treatment of neurological injuries. Lancet 2008, 37, 1955–1969. [Google Scholar] [CrossRef]
- Dietrich, W.D.; Bramlett, H.M. Therapeutic hypothermia and targeted temperature management for traumatic brain injury: Experimental and clinical experience. Brain Circ. 2017, 3, 186–198. [Google Scholar] [CrossRef]
- Dietrich, W.D.; Bramlett, H.M. Therapeutic hypothermia and targeted temperature management in traumatic brain injury: Clinical challenges for successful translation. Brain Res. 2016, 1640, 94–103. [Google Scholar] [CrossRef] [Green Version]
- Walter, A.; Finelli, K.; Bai, X.; Johnson, B.; Neuberger, T.; Seidenberg, P.; Bream, T.; Hallett, M.; Slobounov, S. Neurobiological effect of selective brain cooling after concussive injury. Brain Imaging Behav. 2018, 12, 891–900. [Google Scholar] [CrossRef]
- Foda, M.A.; Marmarou, A. A new model of diffuse brain injury in rats. J. Neurosurg. 1994, 80, 301–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marmarou, A.; Foda, M.A.; van den Brink, W.; Campbell, J.; Kita, H.; Demetriadou, K. A new model of diffuse brain injury in rats. J. Neurosurg. 1994, 80, 291–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCrea, M.; Guskiewicz, K.; Randolph, C.; Barr, W.B.; Hammeke, T.A.; Marshall, S.W.; Kelly, J.P. Effects of symptom-free waiting period on clinical outcome and risk of reinjury after sport-related concussion. Neurosurgery 2009, 65, 876–883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ginsberg, M.D.; Zhao, W.; Alonso, O.F.; Loor-Estades, J.Y.; Dietrich, W.D.; Busto, R. Uncoupling of local cerebral glucose metabolism and blood flow after acute fluid-percussion injury in rats. Am. J. Physiol. 1997, 272, H2859–H2868. [Google Scholar] [CrossRef] [PubMed]
- Sokoloff, L.; Reivich, M.; Kennedy, C.; Des Rosiers, M.H.; Patlak, C.S.; Pettigrew, K.D.; Sakurada, O.; Shinohara, M. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: Theory, procedure, and normal values in the conscious and anesthetized rat. J. Neurochem. 1977, 28, 897–916. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates; Academic Press: New York, NY, USA, 1982. [Google Scholar]
- Zhao, W.; Young, T.Y.; Ginsberg, M.D. Registration and three-dimensional reconstruction of autoradiographic images by the disparity analysis method. IEEE Trans. Med. Imaging 1993, 12, 782–791. [Google Scholar] [CrossRef]
- Zhao, W.; Ginsberg, M.D.; Smith, D.W. Three-dimensional quantitative autoradiography by disparity analysis: Theory and application to image-averaging of local cerebral glucose utilization. J. Cereb. Blood Flow Metab. 1995, 15, 552–565. [Google Scholar] [CrossRef]
- Zhao, W.; Belayev, L.; Ginsberg, M.D. Transient middle cerebral artery occlusion by intraluminal suture: II. Neurological deficits, and pixel-based correlation of histopathology with local blood flow and glucose utilization. J. Cereb. Blood Flow Metab. 1997, 17, 1281–1290. [Google Scholar] [CrossRef] [Green Version]




| TBI #1 (Day 0) | TBI #2 (Day 3) | TBI #3 (Day 7) | 2DG (Day 8) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Weight (g) | Head Temp (°C) | Body Temp (°C) | Weight (g) | Head Temp (°C) | Body Temp (°C) | Weight (g) | Head Temp (°C) | Body Temp (°C) | Weight (g) | |
| Sham | 335 ± 8 | 38.09 ± 0.39 | 38.2 ± 0.35 | 327 ± 5 | 38.14 ± 0.42 | 38.03 ± 0.38 | 348 ± 8 | 38.4 ± 0.36 | 38.3 ± 0.37 | 344 ± 3 |
| Normothermia | 307 ± 3 | 36.85 ± 0.05 | 36.68 ± 0.06 | 304 ± 3 | 36.88 ± 0.04 | 36.73 ± 0.07 | 314 ± 3 | 36.86 ± 0.04 | 36.78 ± 0.05 | 319 ± 4 |
| Hyperthermia | 309 ± 2 | 38.85 ± 0.03 | 38.79 ± 0.06 | 310 ± 4 | 38.84 ± 0.03 | 38.7 ± 0.05` | 313 ± 6 | 38.85 ± 0.02 | 38.56 ± 0.28 | 315 ± 5 |
| Region | Sham | TBI Normo | TBI Hyper | |
|---|---|---|---|---|
| Bregma +0.7 mm | ||||
| Caudate putamen | R | 115.7 ± 6.6 | 92.6 ± 14.1 | 92 ± 12.9 |
| L | 108.1 ± 5.8 | 90.8 ± 12.6 | 80.8 ± 7.3 | |
| Cortical strip | R | 106.3 ± 7.1 | 92.8 ± 12.6 | 84.4 ± 7.4 |
| L | 100.6 ± 4.7 | 83 ± 12.2 | 74.6 ± 5.1 | |
| 1° motor cortex | R | 100.6 ± 9.4 | 77.2 ± 11.6 | 70.6 ± 5.9 |
| L | 95.4 ± 7.2 | 76.7 ± 11.9 | 70.2 ± 6.4 | |
| 2° motor cortex | R | 112 ± 9.6 | 85.1 ± 11.5 | 88.4 ± 9.5 |
| L | 99.6 ± 7.6 | 76.8 ± 12 | 69.4 ± 5.7 | |
| Cingulate cortex | R | 108.5 ± 7 | 87.7 ± 10.8 | 80.8 ± 7.7 |
| L | 98.5 ± 7.4 | 81.7 ± 11.9 | 69 ± 6.9 | |
| Bregma +0.2 mm | ||||
| Caudate putamen | R | 117.1 ± 9.2 | 81.9 ± 12.8 * | 74.9 ± 10 * |
| L | 108.3 ± 8.6 | 78.7 ± 12.3 | 65.9 ± 8.4 * | |
| Cortical strip | R | 114.1 ± 8.1 | 86.5 ±12.4 | 79 ± 8.1 * |
| L | 106.7 ± 6.5 | 77.1 ± 11.7 | 67.5 ± 8.3 * | |
| 1° motor cortex | R | 112.9 ± 9 | 77.1 ± 12.8 * | 68 ± 7.2 ** |
| L | 99.2 ± 7.3 | 75.9 ± 12.3 | 60.9 ± 9 * | |
| 2° motor cortex | R | 127.2 ± 12.8 | 81.6 ± 12.7 ** | 75.4 ± 7.5 ** |
| L | 113.4 ± 5.9 | 79.4 ± 12.9 | 63.9 ± 7.4 ** | |
| Cingulate cortex | R | 133.5 ± 16.3 | 86.3 ± 11.5 ** | 77.8 ± 7.9 *** |
| L | 112.7 ± 11.3 | 76.1 ± 12.5 * | 66.1 ± 5.7 ** | |
| Bregma −0.8 mm | ||||
| Caudate putamen | R | 94.2 ± 7.6 | 76.2 ± 10.2 | 63.7 ± 6.5 |
| L | 88.7 ± 8.3 | 81.1 ± 12 | 64.8 ± 6.4 | |
| Cortical strip | R | 102.2 ± 4.6 | 93.7 ± 12.1 | 72.6 ± 6.6 |
| L | 104.1 ± 5.6 | 92.2 ±13.4 | 73.5 ± 6.8 | |
| 1° motor cortex | R | 118.2 ± 10.6 | 99.1 ± 9.3 | 71.4 ± 9.6 ** |
| L | 98.2 ± 6.4 | 92.5 ± 12.3 | 67.8 ± 9.1 | |
| 2° motor cortex | R | 132 ± 14.6 | 110.6 ± 10.8 | 89.6 ± 10.5 * |
| L | 103.5 ± 6.2 | 99.7 ± 13.8 | 75.7 ± 8.5 | |
| Cingulate cortex | R | 123 ± 18.1 | 106.3 ± 8.8 | 78.5 ± 8.7 ** |
| L | 107 ± 10.3 | 95.9 ± 14.1 | 69 ± 6.9 * | |
| Bregma −1.8 mm | ||||
| Caudate putamen | R | 99 ± 9.2 | 68 ± 7.4 * | 56.9 ±7.5 ** |
| L | 92.5 ± 9.8 | 70.3 ± 7.5 | 57.6 ± 5.1 * | |
| Cortical strip | R | 110.6 ± 9.9 | 79.6 ± 7.7 * | 71.1 ± 5.5 ** |
| L | 100 ± 7.3 | 73.4 ± 6 | 67.2 ± 5 * | |
| 1° motor cortex | R | 114.9 ± 14 | 70.9 ± 6.8 ** | 59.5 ± 5.9 **** |
| L | 100.2 ± 9.3 | 63.8 ± 5 ** | 58.3 ± 2.4 ** | |
| 2° motor cortex | R | 135.3 ± 19.3 | 94.1 ± 5.2 ** | 76.7 ± 6.7 **** |
| L | 121.7 ± 12.2 | 83 ± 7.2 ** | 75.8 ± 7 *** | |
| Cingulate cortex | R | 127.7 ± 16.1 | 81.7 ± 5.8 *** | 68.1 ± 4.8 **** |
| L | 116.3 ± 13.9 | 77.9 ± 7.4 ** | 61.1 ± 5 **** | |
| Bregma −2.8 mm | ||||
| Caudate putamen | R | 68.8 ± 8.2 | 47.6 ± 4.2 | 42 ± 4.3 |
| L | 71.4 ± 8.3 | 54.4 ± 6.3 | 48.8 ± 4.3 | |
| Hippocampus | R | 74.5 ± 5.4 | 53 ± 3.8 | 58.3 ± 4.7 |
| L | 80.9 ± 5.3 | 58.8 ± 4.8 | 60.4 ± 4.6 | |
| Cortical strip | R | 102.9 ± 9.1 | 74.3 ± 6.8 * | 70.4 ± 3.5 * |
| L | 93.8 ± 7.2 | 71.3 ± 5.8 | 67.6 ± 4.3 | |
| 1° motor cortex | R | 104.5 ± 13.5 | 69.8 ± 8.6 ** | 56.9 ± 7.6 *** |
| L | 83.6 ± 9.7 | 66.2 ± 5 | 59.4 ± 5.4 | |
| 2° motor cortex | R | 118.2 ± 16.8 | 84.2 ± 9.2 ** | 69.8 ± 9 **** |
| L | 93 ± 12.6 | 75.9 ± 5.7 | 68 ± 4.7 | |
| Retrosplenial cortex | R | 115.8 ± 19.2 | 80.9 ± 8.3 ** | 69 ± 6.8 *** |
| L | 97 ± 13.2 | 75.1 ± 8.1 | 60.5 ± 4.2 ** | |
| Bregma −3.8 mm | ||||
| Striatum | R | 137 ± 11.4 | 91.9 ± 11.8 ** | 79.2 ± 9.2 *** |
| L | 129.2 ± 13.1 | 89.8 ± 12.1 * | 77.1 ± 8.6 ** | |
| Hippocampus | R | 76.7 ± 4.6 | 57.5 ± 7.1 | 51.9 ± 6 |
| L | 81.2 ± 6.2 | 60.9 ± 7.4 | 53.9 ± 6.8 | |
| Cortical strip | R | 116.4 ± 11.3 | 84 ± 12 | 71.1 ± 8.3 ** |
| L | 112.1 ± 11.1 | 78.2 ± 11.2 | 71.6 ± 7.8 * | |
| Parietal association cortex | R | 103.9 ± 12.8 | 77.3 ± 11.7 | 61.2 ± 8.1 * |
| L | 93.9 ± 10.7 | 74.5 ± 10.9 | 64.9 ± 6.5 | |
| Retrosplenial cortex | R | 102.3 ± 18.7 | 80.6 ± 12.7 | 63.1 ± 7.5 * |
| L | 96.9 ± 15.4 | 77.2 ± 13 | 64.1 ± 6.7 | |
| Bregma −4.8 mm | ||||
| Striatum | R | 110.6 ± 12.1 | 68.2 ± 8.7 * | 64.7 ± 7.5 * |
| L | 107.5 ± 14.8 | 67.1 ± 8.6 * | 65.6 ± 7.8 * | |
| Hippocampus | R | 79.8 ± 9.9 | 52.5 ± 7.5 | 51.4 ± 7.1 |
| L | 89.1 ± 11.8 | 55 ± 6.9 | 52.1 ± 7.7 | |
| Cortical strip | R | 129.5 ± 14.8 | 81 ± 13.4 ** | 78.2 ± 6.7 ** |
| L | 114.1 ± 12.8 | 75.5 ± 12.3 * | 71.2 ± 6 * | |
| Visual cortex | R | 115.1 ± 18.1 | 73.3 ± 10.9 * | 63.6 ± 7 ** |
| L | 120.1 ± 19.6 | 74.4 ± 10.9 * | 67.6 ± 7.4 ** | |
| Retrosplenial cortex | R | 134.1 ± 21.6 | 86.4 ± 12.1 ** | 70.9 ± 8.7 *** |
| L | 116 ± 15.6 | 81.1 ± 13.7 | 69.6 ± 5.4 * | |
| Bregma −5.8 mm | ||||
| Striatum | R | 83.5 ± 7.6 | 66.2 ± 8.9 | 61.5 ± 6.4 |
| L | 84.3 ± 9.1 | 65.3 ± 8.2 | 61.3 ± 7 | |
| Hippocampus | R | 78.9 ± 9.7 | 56.9 ± 6.9 | 56.3 ± 6.3 |
| L | 81.3 ± 8.7 | 57.3 ± 6.3 | 54.4 ± 6.7 | |
| Cortical strip | R | 111.9 ± 14.6 | 78.9 ± 11 * | 76.8 ± 8.4 * |
| L | 103.1 ± 10.7 | 77.1 ± 11.1 | 71.4 ± 9.4 | |
| Visual cortex | R | 95.4 ± 12.3 | 73.4 ± 10.5 | 61.4 ± 9.8 * |
| L | 90.1 ± 11 | 71.6 ± 9 | 63 ± 8.8 | |
| Retrosplenial cortex | R | 102.2 ± 12.7 | 76.3 ± 10.9 | 67.6 ± 8.9 * |
| L | 105.4 ± 9.6 | 81.2 ± 11.4 | 66.1 ± 9.1 * | |
| Bregma −7.3 mm | ||||
| Striatum | R | 83.7 ± 5 | 67.6 ± 7.8 | 55.8 ± 7 |
| L | 91 ± 8.2 | 67 ± 6.7 | 59.4 ± 7.2 * | |
| Cortical strip | R | 118.3 ± 13.6 | 91 ± 9.5 | 78.1 ± 5.4 ** |
| L | 117.4 ± 12.2 | 81.1 ± 10.2 ** | 76.5 ± 6.6 ** | |
| Visual cortex | R | 101.2 ± 8.8 | 88.7 ± 9.8 | 71.7 ± 6.7 * |
| L | 111.5 ± 11.6 | 84.3 ± 9 | 77.9 ± 5.5 * | |
| Retrosplenial cortex | R | 102.6 ± 8.7 | 84.7 ± 8.2 | 72 ± 6.8 * |
| L | 105.2 ± 10.3 | 83 ± 9.3 | 73.6 ± 6.1 * | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blaya, M.; Truettner, J.; Zhao, W.; Bramlett, H.; Dietrich, W.D. Mild Hyperthermia Aggravates Glucose Metabolic Consequences in Repetitive Concussion. Int. J. Mol. Sci. 2020, 21, 609. https://doi.org/10.3390/ijms21020609
Blaya M, Truettner J, Zhao W, Bramlett H, Dietrich WD. Mild Hyperthermia Aggravates Glucose Metabolic Consequences in Repetitive Concussion. International Journal of Molecular Sciences. 2020; 21(2):609. https://doi.org/10.3390/ijms21020609
Chicago/Turabian StyleBlaya, Meghan, Jessie Truettner, Weizhao Zhao, Helen Bramlett, and William Dalton Dietrich. 2020. "Mild Hyperthermia Aggravates Glucose Metabolic Consequences in Repetitive Concussion" International Journal of Molecular Sciences 21, no. 2: 609. https://doi.org/10.3390/ijms21020609
APA StyleBlaya, M., Truettner, J., Zhao, W., Bramlett, H., & Dietrich, W. D. (2020). Mild Hyperthermia Aggravates Glucose Metabolic Consequences in Repetitive Concussion. International Journal of Molecular Sciences, 21(2), 609. https://doi.org/10.3390/ijms21020609