Mechanism of the Affinity-Enhancing Effect of Isatin on Human Ferrochelatase and Adrenodoxin Reductase Complex Formation: Implication for Protein Interactome Regulation
Abstract
:1. Introduction
2. Results
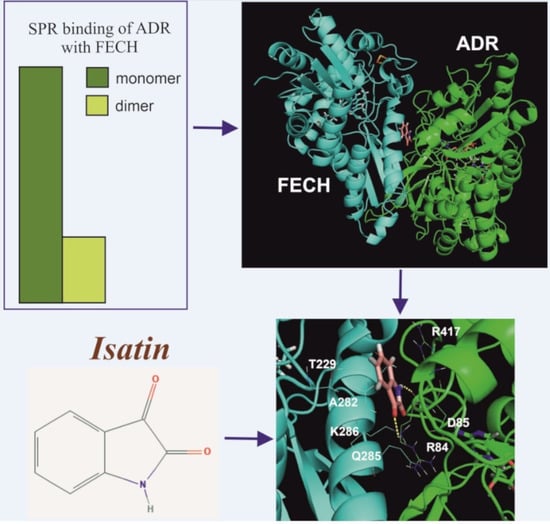
2.1. Interaction of FECH with ADR
2.2. The Effect of Isatin and Its Derivatives on FECH Interaction with Protein Partners
2.3. Molecular Modeling of the Isatin Effect on the Affinity of Protein–protein Interaction
3. Discussion
4. Materials and Methods
4.1. Recombinant Proteins and Chemicals
4.2. Surface Plasmon Resonance (SPR) Analysis
4.2.1. FECH Immobilization on the SPR Chip
4.2.2. Chemical Stabilization of FECH Dimers on the Optical Chip Surface
4.2.3. The SPR Study of Protein–Protein Interactions (PPIs)
4.3. Computer Aided Molecular Modeling and Docking
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| SPR | surface plasmon resonance |
| FECH | ferrochelatase |
| ADR | adrenodoxine reductase |
| PPI | protein–protein interactions |
| CYB5B | cytochrome b5 type B |
| SMAD4 | SMAD family member 4 |
| B2M | beta-2-microglobulin |
| BSA | bovine serum protein |
References
- Medvedev, A.; Buneeva, O.; Gnedenko, O.; Ershov, P.; Ivanov, A. Isatin, an endogenous nonpeptide biofactor: A review of its molecular targets, mechanisms of actions, and their biomedical implications. BioFactors 2018, 44, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Medvedev, A.; Buneeva, O.; Glover, V. Biological targets for isatin and its analogues: Implications for therapy. Biologics 2007, 1, 151–162. [Google Scholar] [PubMed]
- Buneeva, O.; Gnedenko, O.; Zgoda, V.; Kopylov, A.; Glover, V.; Ivanov, A.; Medvedev, A.; Archakov, A. Isatin-binding proteins of rat and mouse brain: Proteomic identification and optical biosensor validation. Proteomics 2010, 10, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Medvedev, A.E.; Buneeva, O.A.; Kopylov, A.T.; Gnedenko, O.V.; Medvedeva, M.V.; Kozin, S.A.; Ivanov, A.S.; Zgoda, V.G.; Makarov, A.A. The effects of endogenous non-peptide molecule isatin and hydrogen peroxide on proteomic profiling of rat brain amyloid-β binding proteins: Relevance to Alzheimer’s disease? Int. J. Mol. Sci. 2014, 16, 476–495. [Google Scholar] [CrossRef] [Green Version]
- Crumeyrolle-Arias, M.; Buneeva, O.; Zgoda, V.; Kopylov, A.; Cardona, A.; Tournaire, M.-C.; Pozdnev, V.; Glover, V.; Medvedev, A. Isatin binding proteins in rat brain: In situ imaging, quantitative characterization of specific [3H]isatin binding, and proteomic profiling. J. Neurosci. Res. 2009, 87, 2763–2772. [Google Scholar] [CrossRef]
- Buneeva, O.A.; Gnedenko, O.V.; Medvedeva, M.V.; Ivanov, A.S.; Medvedev, A.E. Oxidative modification of glyceraldehyde-3-phosphate dehydrogenase influences its interaction with endogenous neuroprotector isatin. Biochem. Mosc. Suppl. Ser. B 2015, 9, 185–188. [Google Scholar] [CrossRef]
- Medvedev, A.; Buneeva, O.; Gnedenko, O.; Fedchenko, V.; Medvedeva, M.; Ivanov, Y.; Glover, V.; Sandler, M. Isatin interaction with glyceraldehyde-3-phosphate dehydrogenase, a putative target of neuroprotective drugs: Partial agonism with deprenyl. J. Neural Transm. 2006, (Suppl. 71), 97–103. [Google Scholar] [CrossRef]
- Ershov, P.; Mezentsev, Y.; Gilep, A.; Usanov, S.; Buneeva, O.; Medvedev, A.; Ivanov, A. Isatin-induced increase in the affinity of human ferrochelatase and adrenodoxin reductase interaction. Protein Sci. 2017, 26, 2458–2462. [Google Scholar] [CrossRef]
- Svirid, A.V.; Ershov, P.V.; Yablokov, E.O.; Kaluzhskiy, L.A.; Mezentsev, Y.V.; Florinskaya, A.V.; Sushko, T.A.; Strushkevich, N.V.; Gilep, A.A.; Usanov, S.A.; et al. Direct Molecular Fishing of New Protein Partners for Human Thromboxane Synthase. Acta Nat. 2017, 9, 92–100. [Google Scholar] [CrossRef]
- Burden, A.E.; Wu, C.; Dailey, T.A.; Busch, J.L.; Dhawan, I.K.; Rose, J.P.; Wang, B.; Dailey, H.A. Human ferrochelatase: Crystallization, characterization of the [2Fe-2S] cluster and determination that the enzyme is a homodimer. Biochim. Biophys. Acta 1999, 1435, 191–197. [Google Scholar] [CrossRef]
- Wu, C.K.; Dailey, H.A.; Rose, J.P.; Burden, A.; Sellers, V.M.; Wang, B.C. The 2.0 A structure of human ferrochelatase, the terminal enzyme of heme biosynthesis. Nat. Struct. Biol. 2001, 8, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, A.S.; Medvedev, A.; Ershov, P.; Molnar, A.; Mezentsev, Y.; Yablokov, E.; Kaluzhsky, L.; Gnedenko, O.; Buneeva, O.; Haidukevich, I.; et al. Protein interactomics based on direct molecular fishing on paramagnetic particles: Practical realization and further SPR validation. Proteomics 2014, 14, 2261–2274. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, A.S.; Ershov, P.V.; Molnar, A.A.; Yu, M.; Kaluzhskiy, L.A.; Yablokov, E.O.; Florinskaya, A.V.; Gnedenko, O.V.; Medvedev, A.E.; Kozin, S.A.; et al. Direct molecular fishing in molecular partners investigation in protein-protein and protein-peptide interactions. Russ. J. Bioorganic Chem. 2016, 42, 14–21. [Google Scholar] [CrossRef]
- Ivanov, A.S.; Medvedev, A.E.; Buneeva, O.A.; Gnedenko, O.V.; Ershov, P.V.; Mezentsev, Y.V.; Yablokov, E.O.; Kaluzhskiy, L.A.; Florinskaya, A.V.; Moskaleva, N.E.; et al. The influence of gravity discharge on the content of isatin-binding proteins in mice: The results of ground-based and space research under the program BION-M no. 1. Biochem. (Mosc.) Suppl. Ser. B. Biomed. Chem. 2016, 10, 227–229. [Google Scholar] [CrossRef]
- Ershov, P.V.; Gnedenko, O.V.; Molnar, A.A.; Lisitsa, A.V.; Ivanov, A.S.; Archakov, A.I. Biosensor analysis of the interaction of potential dimerization inhibitors with HIV-1 protease. Biochem. Mosc. Suppl. Ser. B. 2009, 3, 272–288. [Google Scholar] [CrossRef]
- Ershov, P.V.; Gnedenko, O.V.; Molnar, A.A.; Lisitsa, A.V.; Ivanov, A.S.; Archakov, A.I. Kinetic and thermodynamic analysis of dimerization inhibitors binding to HIV protease monomers by surface plasmon resonance. Biochem. Mosc. Suppl. Ser. B. 2012, 6, 94–97. [Google Scholar] [CrossRef]
- Ershov, P.V.; Mezentsev, Y.V.; Yablokov, E.O.; Kaluzhskiy, L.A.; Florinskaya, A.V.; Svirid, A.V.; Gilep, A.A.; Usanov, S.A.; Medvedev, A.E.; Ivanov, A.S. Specificity of Isatin Interaction with Cytochromes P450. Biochem. (Mosc.) Suppl. Ser. B: Biomed. Chem. 2018, 12, 130–135. [Google Scholar] [CrossRef]
- Medvedev, A.; Kopylov, A.; Buneeva, O.; Kurbatov, L.; Tikhonova, O.; Ivanov, A.; Zgoda, V. A Neuroprotective Dose of Isatin Causes Multilevel Changes Involving the Brain Proteome: Prospects for Further Research. Int. J. Mol. Sci. 2020, 21, 4187. [Google Scholar] [CrossRef]
- Müller, J.J.; Lapko, A.; Bourenkov, G.; Ruckpaul, K.; Heinemann, U. Adrenodoxin reductase-adrenodoxin complex structure suggests electron transfer path in steroid biosynthesis. J. Biol. Chem. 2001, 276, 2786–2789. [Google Scholar] [CrossRef] [Green Version]
- Söderberg, C.; Gillam, M.E.; Ahlgren, E.-C.; Hunter, G.A.; Gakh, O.; Isaya, G.; Ferreira, G.C.; Al-Karadaghi, S. The Structure of the Complex between Yeast Frataxin and Ferrochelatase: Characterization and Pre-Steady State Reaction of Ferrous Iron Delivery and Heme Synthesis. J. Biol. Chem. 2016, 291, 11887–11898. [Google Scholar] [CrossRef] [Green Version]
- Piel, R.B.; Shiferaw, M.T.; Vashisht, A.A.; Marcero, J.R.; Praissman, J.L.; Phillips, J.D.; Wohlschlegel, J.A.; Medlock, A.E. A Novel Role for Progesterone Receptor Membrane Component 1 (PGRMC1): A Partner and Regulator of Ferrochelatase. Biochemistry 2016, 55, 5204–5217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, T.; Grimm, B.; Layer, G. Chapter Four—Porphyrin and heme synthesis. Metabolism, Structure and Function of Plant Tetrapyrroles: Control Mechanisms of Chlorophyll Biosynthesis and Analysis of Chlorophyll-Binding Proteins. In Advances in Botanical Research, Grimm, B., Ed.; Academic Press: Cambridge, MA, USA, 2019; Volume 91, pp. 89–131. [Google Scholar]
- Shi, Y.; Ghosh, M.; Kovtunovych, G.; Crooks, D.R.; Rouault, T.A. Both human ferredoxins 1 and 2 and ferredoxin reductase are important for iron-sulfur cluster biogenesis. Biochim. Et Biophys. Acta Mol. Cell Res. 2012, 1823, 484–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Napoli, E.; Morin, D.; Bernhardt, R.; Buckpitt, A.; Cortopassi, G. Hemin rescues adrenodoxin, heme a and cytochrome oxidase activity in frataxin-deficient oligodendroglioma cells. Biochim. Biophys. Acta Mol. Basis Dis. 2007, 1772, 773–780. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, G.C.; Franco, R.; Lloyd, S.G.; Moura, I.; Moura, J.J.; Huynh, B.H. Structure and function of ferrochelatase. J. Bioenerg. Biomembr. 1995, 27, 221–229. [Google Scholar] [CrossRef]
- Hurley, J.K.; Weber-Main, A.M.; Hodges, A.E.; Stankovich, M.T.; Benning, M.M.; Holden, H.M.; Cheng, H.; Xia, B.; Markley, J.L.; Genzor, C.; et al. Iron-sulfur cluster cysteine-to-serine mutants of Anabaena -2Fe-2S- ferredoxin exhibit unexpected redox properties and are competent in electron transfer to ferredoxin:NADP+ reductase. Biochemistry 1997, 36, 15109–15117. [Google Scholar] [CrossRef] [PubMed]
- Mitani, F.; Ishimura, Y.; Izumi, S.; Watanabe, K. Immunohistochemical localization of adrenodoxin and adrenodoxin reductase in bovine adrenal cortex. Acta Endocrinol. 1979, 90, 317–327. [Google Scholar] [CrossRef]
- Sakaino, M.; Ishigaki, M.; Ohgari, Y.; Kitajima, S.; Masaki, R.; Yamamoto, A.; Taketani, S. Dual mitochondrial localization and different roles of the reversible reaction of mammalian ferrochelatase. FEBS J. 2009, 276, 5559–5570. [Google Scholar] [CrossRef]
- Sergeev, G.V.; Gilep, A.A.; Usanov, S.A. The role of cytochrome b5 structural domains in interaction with cytochromes P450. Biochem. Mosc. 2014, 79, 406–416. [Google Scholar] [CrossRef]
- Gilep, A.A.; Guryev, O.L.; Usanov, S.A.; Estabrook, R.W. Apo-cytochrome b5 as an indicator of changes in heme accessability: Preliminary studies with cytochrome P450 3A4. J. Inorg. Biochem. 2001, 87, 237–244. [Google Scholar] [CrossRef]
- Usanov, S.A.; Graham, S.E.; Lepesheva, G.I.; Azeva, T.N.; Strushkevich, N.V.; Gilep, A.A.; Estabrook, R.W.; Peterson, J.A. Probing the Interaction of Bovine Cytochrome P450scc (CYP11A1) with Adrenodoxin: Evaluating Site-Directed Mutations by Molecular Modeling. Biochemistry 2002, 41, 8310–8320. [Google Scholar] [CrossRef]
- Medvedev, A.E.; Goodwin, B.L.; Sandler, M.; Glover, V. Efficacy of isatin analogues as antagonists of rat brain and heart atrial natriuretic peptide receptors coupled to particulate guanylyl cyclase. Biochem. Pharmacol. 1999, 57, 913–915. [Google Scholar] [CrossRef]
- Markgren, P.O.; Hämäläinen, M.; Danielson, U.H. Screening of compounds interacting with HIV-1 proteinase using optical biosensor technology. Anal. Biochem. 1998, 265, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Vajda, S.; Yueh, C.; Beglov, D.; Bohnuud, T.; Mottarella, S.E.; Xia, B.; Hall, D.R.; Kozakov, D. New additions to the ClusPro server motivated by CAPRI. Proteins 2017, 85, 435–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tovchigrechko, A.; Vakser, I.A. GRAMM-X public web server for protein-protein docking. Nucleic Acids Res. 2006, 34, W310–W314. [Google Scholar] [CrossRef]
- Pierce, B.G.; Wiehe, K.; Hwang, H.; Kim, B.-H.; Vreven, T.; Weng, Z. ZDOCK server: Interactive docking prediction of protein-protein complexes and symmetric multimers. Bioinformatics 2014, 30, 1771–1773. [Google Scholar] [CrossRef]
- Allen, W.J.; Balius, T.E.; Mukherjee, S.; Brozell, S.R.; Moustakas, D.T.; Lang, P.T.; Case, D.A.; Kuntz, I.D.; Rizzo, R.C. DOCK 6: Impact of new features and current docking performance. J. Comput. Chem 2015, 36, 1132–1156. [Google Scholar] [CrossRef] [Green Version]






| Compound Number | Name/CAS/Empirical Formula/Molecular Weight, Da | Structure |
|---|---|---|
| 0 | Isatin/91-56-5/C8H5NO2/147 |  |
| 1 | 5-Methylisatin/608-05-9/C9H7NO2/161 |  |
| 2 | 5-Bromoisatin/87-48-9/C8H4BrNO2/226 |  |
| 3 | 5-Iodoisatin/20780-76-1/C8H4INO2/273 |  |
| 4 | 5-Fluoroisatin/443-69-6/C8H4FNO2/165 |  |
| 5 | 5-Nitroisatin/611-09-6/C8H4N2O4/192 |  |
| 6 | 5,7-Dichloroisatin/6374-92-1/C8H3Cl2NO2/216 |  |
| 7 | 3-hydroxy-3-(2-oxopropyl)indolin-2-one/C11H11NO3/205 |  |
| Control * | №0 ** | №1 *** | №2 | №3 | №4 | №5 | №6 | №7 |
|---|---|---|---|---|---|---|---|---|
| FECH/ADR | ||||||||
| 15.2 ± 1.2 | 3.4 ± 0.4 | 14.3 ± 1.2 | 15.5 ± 1.5 | 14.1 ± 0.8 | 13.8 ± 1.4 | 13.7 ± 0.9 | 14.7 ± 1.4 | 15.5 ± 0.8 |
| FECH/FECH | ||||||||
| 0.55 ± 0.11 | 0.60 ± 0.05 | 0.57 ± 0.06 | 0.53 ± 0.06 | 0.54 ± 0.05 | 0.61 ± 0.06 | 0.56 ± 0.08 | 0.58 ± 0.06 | 0.53 ± 0.10 |
| N of Complex | Balanced Scoring Function | N of Complex | Hydrophobic Scoring Function |
|---|---|---|---|
| 1 | −657.9 | 1 | −931.3 |
| 2 | −705.0 | 2 | −910.8 |
| 3 | −737.1 | 3 | −808.0 |
| 4 | −680.9 | 4 | −986.8 |
| 5 | −656.6 | 5 | −815,3 |
| 6 | −672.8 | 6 | −922.9 |
| 7 | −680.5 | 7 | −830.9 |
| 8 | −740.7 | 8 | −914.8 |
| 9 | −742,9 | 9 | −915.6 |
| 10 | −673.6 | 10 | −882.2 |
| Biosensor Channel | FECH Immobilization | Solution Injection | ||
|---|---|---|---|---|
| 5 мM NaOH | FECH | NHS + EDC | ||
| Fc1 (control 1) | - | - | - | - |
| Fc2 (monomer form) | + | + | - | - |
| Fc3 (control 2) | + | + | - | + |
| Fc4 (dimer form) | + | + | + | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ershov, P.V.; Veselovsky, A.V.; Mezentsev, Y.V.; Yablokov, E.O.; Kaluzhskiy, L.A.; Tumilovich, A.M.; Kavaleuski, A.A.; Gilep, A.A.; Moskovkina, T.V.; Medvedev, A.E.; et al. Mechanism of the Affinity-Enhancing Effect of Isatin on Human Ferrochelatase and Adrenodoxin Reductase Complex Formation: Implication for Protein Interactome Regulation. Int. J. Mol. Sci. 2020, 21, 7605. https://doi.org/10.3390/ijms21207605
Ershov PV, Veselovsky AV, Mezentsev YV, Yablokov EO, Kaluzhskiy LA, Tumilovich AM, Kavaleuski AA, Gilep AA, Moskovkina TV, Medvedev AE, et al. Mechanism of the Affinity-Enhancing Effect of Isatin on Human Ferrochelatase and Adrenodoxin Reductase Complex Formation: Implication for Protein Interactome Regulation. International Journal of Molecular Sciences. 2020; 21(20):7605. https://doi.org/10.3390/ijms21207605
Chicago/Turabian StyleErshov, Pavel V., Alexander V. Veselovsky, Yuri V. Mezentsev, Evgeniy O. Yablokov, Leonid A. Kaluzhskiy, Anastasiya M. Tumilovich, Anton A. Kavaleuski, Andrei A. Gilep, Taisiya V. Moskovkina, Alexei E. Medvedev, and et al. 2020. "Mechanism of the Affinity-Enhancing Effect of Isatin on Human Ferrochelatase and Adrenodoxin Reductase Complex Formation: Implication for Protein Interactome Regulation" International Journal of Molecular Sciences 21, no. 20: 7605. https://doi.org/10.3390/ijms21207605
APA StyleErshov, P. V., Veselovsky, A. V., Mezentsev, Y. V., Yablokov, E. O., Kaluzhskiy, L. A., Tumilovich, A. M., Kavaleuski, A. A., Gilep, A. A., Moskovkina, T. V., Medvedev, A. E., & Ivanov, A. S. (2020). Mechanism of the Affinity-Enhancing Effect of Isatin on Human Ferrochelatase and Adrenodoxin Reductase Complex Formation: Implication for Protein Interactome Regulation. International Journal of Molecular Sciences, 21(20), 7605. https://doi.org/10.3390/ijms21207605