Development of a Method for Scaffold-Free Elastic Cartilage Creation
Abstract
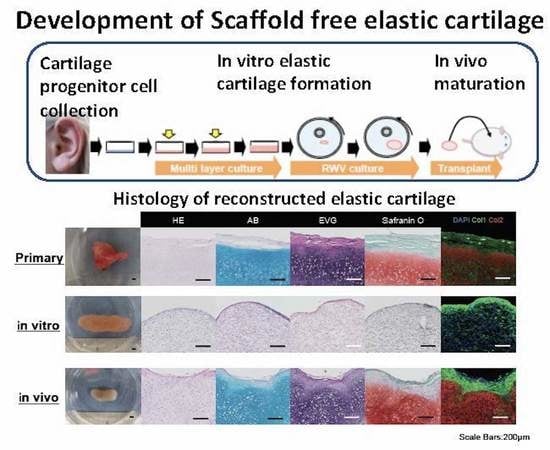
:1. Introduction
2. Results
2.1. Isolation of Cartilage Progenitor Cells
2.2. Generation of Cultured Cartilage for Multilayer and Rotating Cultures
2.3. Maturation of Transplanting Cultured Cartilage
2.4. Long-Term Changes of the Cartilage Tissue after Transplantation
3. Discussion
4. Materials and Methods
4.1. Study Approval
4.2. Animals
4.3. Isolation of Chondrocyte Progenitor Cells and Culture Method
4.4. Multilayer Culture
4.5. RWV Culture
4.6. Cultured Elastic Cartilage Tissue Transplantation to NOD/SCID Mice
4.7. Measurement of Transplanted Elastic Cartilage Tissue and Harvest Method
4.8. Histochemistry and Immunohistochemistry
4.9. MIA ELISA
4.10. Glycosaminoglycan Assay
4.11. Measurement of Shear Stress
4.12. Statistics
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AB | Alcian blue |
| EVG | Elastica–van Gieson |
| HE | Hematoxylin and eosin |
| MIA | Melanoma-inhibiting activity |
| Col1 | Collagen type Ⅰ |
| Col2 | Collagen type Ⅱ |
| RWV | Rotating wall vessel |
References
- Chang, S.C.N.; Tobias, G.; Roy, A.K.; Vacanti, C.A.; Bonassar, L.J. Tissue engineering of autologous cartilage for craniofacial reconstruction by injection molding. Plast. Reconstr. Surg. 2003, 112, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Takebe, T.; Inui, M.; Iwai, S.; Kan, H.; Zheng, Y.W.; Maegawa, J.; Taniguchi, H. Reconstruction of human elastic cartilage by a CD44+ CD90+ stem cell in the ear perichondrium. Proc. Natl. Acad. Sci. USA 2011, 108, 14479–14484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, S.; Takebe, T.; Zheng, Y.W.; Mizuno, M.; Yabuki, Y.; Maegawa, J.; Taniguchi, H. Presence of cartilage stem/progenitor cells in adult mice auricular perichondrium. PLoS ONE 2011, 6, e26393. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Vacanti, J.P.; Paige, J.P.; Upton, J.; Vacanti, C.A. Transplantation of chondrocytes utilizing a polymer-cell construct to produce tissue-engineered cartilage in the shape of a human ear. Plast. Reconstr. Surg. 1997, 100, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Fulco, I.; Miot, S.; Haug, M.D.; Barbero, A.; Wixmerten, A.; Feliciano, S.; Wolf, F.; Jundt, G.; Marsano, A.; Farhadi, J.; et al. Engineered autologous cartilage tissue for nasal reconstruction after tumor resection. An observational first-in-human trial. Clin. Trial 2014, 384, 337–346. [Google Scholar]
- Liu, Y.; Zhang, L.; Zhou, G.; Li, Q.; Liu, W.; Yu, Z.; Luo, X.; Jiang, T.; Zhang, W.; Cao, Y. In vitro engineering of human ear-shaped cartilage assisted with CAD/CAM technology. Biomaterials 2010, 31, 2176–2183. [Google Scholar] [CrossRef]
- Reiffel, A.J.; Kafka, C.; Hernandez, K.A.; Popa, S.; Perez, J.L.; Zhou, S.; Pramanik, S.; Brown, B.N.; Ryu, W.S.; Bonassar, L.J.; et al. High-fidelity tissue engineering of patient-specific auricles for reconstruction of pediatric microtia and other auricular deformities. PLoS ONE 2013, 8, e56506. [Google Scholar] [CrossRef] [Green Version]
- Shieh, S.J.; Terada, S.; Vacanti, J.P. Tissue engineering auricular reconstruction: In vitro and in vivo studies. Biomaterials 2004, 25, 1545–1557. [Google Scholar] [CrossRef]
- Jessop, Z.M.; Javed, M.; Otto, I.A.; Combellack, E.J.; Morgan, S.; Breugem, C.C.; Archer, C.W.; Khan, I.M.; Lineaweaver, W.C.; Kon, M.; et al. Combining regenerative medicine strategies to provide durable reconstructive options: Auricular cartilage tissue engineering. Stem Cell Res. Ther. 2016, 7, 19. [Google Scholar] [CrossRef] [Green Version]
- Nimeskern, L.; Avila, H.M.; Sundberg, J.; Gatenholm, P.; Muller, R.; Stok, K.S. Mechanical evaluation of bacterial nanocellulose as an implant material for ear cartilage replacement. J. Mech. Behav. Biomed. Mater. 2013, 22, 12–21. [Google Scholar] [CrossRef] [Green Version]
- Schulze-Tanzil, G.; Mobasheri, A.; Souza, P.; John, T.; Shakibaei, M. Loss of chondrogenic potential in dedifferentiated chondrocytes correlates with deficient Shc-Erk interaction and apoptosis. Osteoarthr. Cart. 2004, 12, 448–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giardini-Rosa, R.; Joazerio, P.P.; Thomas, K.; Collavino, K.; Weber, J.; Waldman, S.D. Development of scaffold-free elastic cartilaginous constructs with structural similarities to auricular cartilage. Tissue Eng. Part. A 2014, 20, 1012–1026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takebe, T.; Kobayashi, S.; Kan, H.; Suzuki, H.; Yabuki, Y.; Mizuno, M.; Adegawa, T.; Yoshioka, T.; Tanaka, J.; Maegawa, J.; et al. Elastic cartilage engineering from cartilage progenitor cells using rotating wall vessel bioreactor. Transplant. Proc. 2012, 44, 1158–1161. [Google Scholar] [CrossRef] [PubMed]
- Ohyabu, Y.; Tanaka, J.; Ikada, Y.; Uemura, T. Cartilage tissue regeneration from bone marrow cells by RWV bioreactor using collagen sponge scaffold. Mater. Sci. Eng. C 2009, 29, 1150–1155. [Google Scholar] [CrossRef]
- Darling, E.M.; Athanasiou, K.A. Rapid phenotypic changes in passaged articular chondrocyte subpopulations. J. Orthop. Res. 2005, 23, 425–432. [Google Scholar] [CrossRef]
- Darling, E.M.; Athanasiou, K.A. Retaining zonal chondrocyte phenotype by means of novel growth environments. Tissue Eng. 2005, 11, 395–403. [Google Scholar] [CrossRef]
- Wagner, W.; Ho, A.D.; Zenke, M. Different facets of aging in human mesenchymal stem cells. Tissue Eng. Part. B Rev. 2010, 16, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Ohyabu, Y.; Kida, N.; Kojima, H.; Taguchi, T.; Tanaka, J.; Uemura, T. Cartilaginous tissue formation from bone marrow cells using rotating wall vessel (RWV) bioreactor. Biotechnol. Bioeng. 2006, 95, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- Brenner, J.M.; Kunz, M.; Tse, M.Y.; Winterborn, A.; Bardana, D.D.; Pang, S.C.; Waldman, S.D. Development of large engineered cartilage constructs from a small population of cells. Biotechnol. Prog. 2013, 29, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Suits, J.M.T.; Kandel, R.A.; Waldman, S.D. The effect of continuous culture on the growth and structure of tissue-engineered cartilage. Biotechnol. Prog. 2009, 25, 508–515. [Google Scholar] [CrossRef]
- Khan, A.A.; Surrao, D.C. The importance of bicarbonate and nonbicarbonate buffer systems in batch and continuous flow bioreactors for articular cartilage tissue engineering. Tissue Eng. Part. C Methods 2012, 18, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Jiang, H.; Yin, Z.; Liu, Y.; Zhang, Q.; Zhang, C.; Pan, B.; Zhou, J.; Zhou, X.; Sun, H.; et al. In vitro regeneration of patient-specific ear-shaped cartilage and its first clinical application for auricular reconstruction. EBioMedicine 2018, 28, 287–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yanaga, H.; Imai, K.; Fujimoto, T.; Yanaga, K. Generating ears from cultured autologous auricular chondrocytes by using two-stage implantation in treatment of microtia. Plast. Reconstr. Surg. 2009, 124, 817–825. [Google Scholar] [CrossRef] [PubMed] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Enomura, M.; Murata, S.; Terado, Y.; Tanaka, M.; Kobayashi, S.; Oba, T.; Kagimoto, S.; Yabuki, Y.; Morita, K.; Uemura, T.; et al. Development of a Method for Scaffold-Free Elastic Cartilage Creation. Int. J. Mol. Sci. 2020, 21, 8496. https://doi.org/10.3390/ijms21228496
Enomura M, Murata S, Terado Y, Tanaka M, Kobayashi S, Oba T, Kagimoto S, Yabuki Y, Morita K, Uemura T, et al. Development of a Method for Scaffold-Free Elastic Cartilage Creation. International Journal of Molecular Sciences. 2020; 21(22):8496. https://doi.org/10.3390/ijms21228496
Chicago/Turabian StyleEnomura, Masahiro, Soichiro Murata, Yuri Terado, Maiko Tanaka, Shinji Kobayashi, Takayoshi Oba, Shintaro Kagimoto, Yuichiro Yabuki, Kenichi Morita, Toshimasa Uemura, and et al. 2020. "Development of a Method for Scaffold-Free Elastic Cartilage Creation" International Journal of Molecular Sciences 21, no. 22: 8496. https://doi.org/10.3390/ijms21228496
APA StyleEnomura, M., Murata, S., Terado, Y., Tanaka, M., Kobayashi, S., Oba, T., Kagimoto, S., Yabuki, Y., Morita, K., Uemura, T., Maegawa, J., & Taniguchi, H. (2020). Development of a Method for Scaffold-Free Elastic Cartilage Creation. International Journal of Molecular Sciences, 21(22), 8496. https://doi.org/10.3390/ijms21228496