FTIR Spectroscopy to Reveal Lipid and Protein Changes Induced on Sperm by Capacitation: Bases for an Improvement of Sample Selection in ART
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sperm Preparation
2.2. FTIR Spectroscopy
2.3. FTIR Data Processing and Analysis
2.4. UV Resonant Raman (UVRR)
3. Results
3.1. Spermiogram
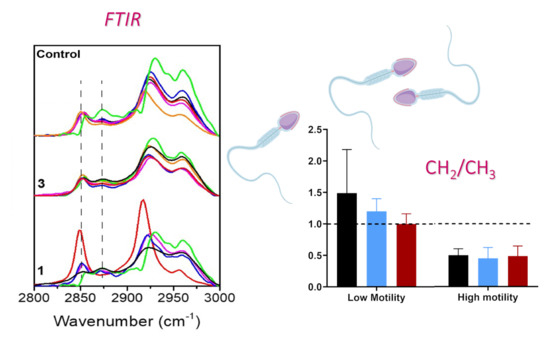
3.2. FTIR Analysis
3.2.1. Protein Secondary Structure
3.2.2. Oxidative Stress: Lipid Peroxidation
3.3. Quantification of DNA Damaging by UV-RAMAN
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ferraretti, A.P.; La Marca, A.; Fauser, B.C.J.M.; Tarlatzis, B.; Nargund, G.; Gianaroli, L. ESHRE working group on Poor Ovarian Response Definition ESHRE consensus on the definition of “poor response” to ovarian stimulation for in vitro fertilization: The Bologna criteria. Hum. Reprod. 2011, 26, 1616–1624. [Google Scholar] [CrossRef] [Green Version]
- Sakkas, D.; Ramalingam, M.; Garrido, N.; Barratt, C.L.R. Sperm selection in natural conception: What can we learn from Mother Nature to improve assisted reproduction outcomes? Hum. Reprod. Update 2015, 21, 711–726. [Google Scholar] [CrossRef] [Green Version]
- Pascolo, L.; Zupin, L.; Gianoncelli, A.; Giolo, E.; Luppi, S.; Martinelli, M.; De Rocco, D.; Sala, S.; Crovella, S.; Ricci, G. XRF analyses reveal that capacitation procedures produce changes in magnesium and copper levels in human sperm. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2019, 459, 120–124. [Google Scholar] [CrossRef]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen, 5th ed.; WHO: Geneva, Switzerland, 2010. [Google Scholar]
- Saleh, R.A.; Agarwal, A.; Sharma, R.K.; Said, T.M.; Sikka, S.C.; Thomas, A.J. Evaluation of nuclear DNA damage in spermatozoa from infertile men with varicocele. Fertil. Steril. 2003, 80, 1431–1436. [Google Scholar] [CrossRef]
- Fernández-Gonzalez, R.; Moreira, P.N.; Pérez-Crespo, M.; Sánchez-Martín, M.; Ramirez, M.A.; Pericuesta, E.; Bilbao, A.; Bermejo-Alvarez, P.; de Hourcade, J.D.; de Fonseca, F.R.; et al. Long-Term Effects of Mouse Intracytoplasmic Sperm Injection with DNA-Fragmented Sperm on Health and Behavior of Adult Offspring1. Biol. Reprod. 2008, 78, 761–772. [Google Scholar] [CrossRef] [Green Version]
- Aitken, R.; Smith, T.; Jobling, M.; Baker, M.; De Iuliis, G. Oxidative stress and male reproductive health. Asian J. Androl. 2014, 16, 31. [Google Scholar] [CrossRef]
- Zhang, Y.; Trussell, J.; Chohan, K. Detecting and Minimizing Sperm DNA Damage. Semin. Reprod. Med. 2013, 31, 267–273. [Google Scholar] [CrossRef]
- Pascolo, L.; Bedolla, D.E.; Vaccari, L.; Venturin, I.; Cammisuli, F.; Gianoncelli, A.; Mitri, E.; Giolo, E.; Luppi, S.; Martinelli, M.; et al. Pitfalls and promises in FTIR spectromicroscopy analyses to monitor iron-mediated DNA damage in sperm. Reprod. Toxicol. 2016, 61, 39–46. [Google Scholar] [CrossRef]
- Mallidis, C.; Sanchez, V.; Wistuba, J.; Wuebbeling, F.; Burger, M.; Fallnich, C.; Schlatt, S. Raman microspectroscopy: Shining a new light on reproductive medicine. Hum. Reprod. Update 2014, 20, 403–414. [Google Scholar] [CrossRef] [Green Version]
- Amaral, S.; Da Costa, R.; Wübbeling, F.; Redmann, K.; Schlatt, S. Raman micro-spectroscopy analysis of different sperm regions: A species comparison. MHR Basic Sci. Reprod. Med. 2018, 24, 185–202. [Google Scholar] [CrossRef]
- D’Amico, F.; Zucchiatti, P.; Latella, K.; Pachetti, M.; Gessini, A.; Masciovecchio, C.; Vaccari, L.; Pascolo, L. Investigation of genomic DNA methylation by UV Resonant Raman spectroscopy. J. Biophotonics 2020. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, F.; Cammisuli, F.; Addobbati, R.; Rizzardi, C.; Gessini, A.; Masciovecchio, C.; Rossi, B.; Pascolo, L. Oxidative damage in DNA bases revealed by UV resonant Raman spectroscopy. Analyst 2015, 140, 1477–1485. [Google Scholar] [CrossRef] [PubMed]
- Portaccio, M.; Errico, S.; Chioccarelli, T.; Cobellis, G.; Lepore, M. Fourier-Transform Infrared Microspectroscopy (FT-IR) Study on Caput and Cauda Mouse Spermatozoa. Proceedings 2019, 42, 19. [Google Scholar] [CrossRef] [Green Version]
- Bambery, K.R.; Wood, B.R.; McNaughton, D. Resonant Mie scattering (RMieS) correction applied to FTIR images of biological tissue samples. Analyst 2012, 137, 126–132. [Google Scholar] [CrossRef]
- Miller, S.A.; Dykes, D.D.; Polesky, H.F. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988, 16, 1215. [Google Scholar] [CrossRef] [Green Version]
- D’Amico, F.; Saito, M.; Bencivenga, F.; Marsi, M.; Gessini, A.; Camisasca, G.; Principi, E.; Cucini, R.; Di Fonzo, S.; Battistoni, A.; et al. UV resonant Raman scattering facility at Elettra. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2013, 703, 33–37. [Google Scholar] [CrossRef]
- Chalmers, J.M.; Griffiths, P.R. (Eds.) Handbook of Vibrational Spectroscopy; John Wiley: New York, NY, USA, 2002; ISBN 978-0-471-98847-2. [Google Scholar]
- Barth, A. Infrared spectroscopy of proteins. Biochim. Biophys. Acta (BBA)-Bioenerg. 2007, 1767, 1073–1101. [Google Scholar] [CrossRef] [Green Version]
- Benedetti, E.; Bramanti, E.; Papineschi, F.; Rossi, I.; Benedetti, E. Determination of the Relative Amount of Nucleic Acids and Proteins in Leukemic and Normal Lymphocytes by Means of Fourier Transform Infrared Microspectroscopy. Appl. Spectrosc. 1997, 51, 792–797. [Google Scholar] [CrossRef]
- Yushchenko, T.; Deuerling, E.; Hauser, K. Insights into the Aggregation Mechanism of PolyQ Proteins with Different Glutamine Repeat Lengths. Biophys. J. 2018, 114, 1847–1857. [Google Scholar] [CrossRef] [Green Version]
- Zandomeneghi, G.; Krebs, M.R.H.; McCammon, M.G.; Fändrich, M. FTIR reveals structural differences between native β-sheet proteins and amyloid fibrils. Protein Sci. 2009, 13, 3314–3321. [Google Scholar] [CrossRef] [Green Version]
- Ami, D.; Lavatelli, F.; Rognoni, P.; Palladini, G.; Raimondi, S.; Giorgetti, S.; Monti, L.; Doglia, S.M.; Natalello, A.; Merlini, G. In situ characterization of protein aggregates in human tissues affected by light chain amyloidosis: A FTIR microspectroscopy study. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, Y.; Li, Y.; Hao, W.; Hu, X.; Ma, G. Parallel β-Sheet Fibril and Antiparallel β-Sheet Oligomer: New Insights into Amyloid Formation of Hen Egg White Lysozyme under Heat and Acidic Condition from FTIR Spectroscopy. J. Phys. Chem. B 2013, 117, 4003–4013. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yang, S.; Kong, J.; Dong, A.; Yu, S. Obtaining information about protein secondary structures in aqueous solution using Fourier transform IR spectroscopy. Nat. Protoc. 2015, 10, 382–396. [Google Scholar] [CrossRef] [PubMed]
- Toyran, N.; Turan, B.; Severcan, F. Selenium alters the lipid content and protein profile of rat heart: An FTIR microspectroscopic study. Arch. Biochem. Biophys. 2007, 458, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Toyran, N.; Lasch, P.; Naumann, D.; Turan, B.; Severcan, F. Early alterations in myocardia and vessels of the diabetic rat heart: An FTIR microspectroscopic study. Biochem. J. 2006, 397, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, G.; Togan, I.; Severcan, F. 17β-Estradiol induced compositional, structural and functional changes in rainbow trout liver, revealed by FT-IR spectroscopy: A comparative study with nonylphenol. Aquat. Toxicol. 2006, 77, 53–63. [Google Scholar] [CrossRef]
- Baker, M.J.; Trevisan, J.; Bassan, P.; Bhargava, R.; Butler, H.J.; Dorling, K.M.; Fielden, P.R.; Fogarty, S.W.; Fullwood, N.J.; Heys, K.A.; et al. Using Fourier transform IR spectroscopy to analyze biological materials. Nat. Protoc. 2014, 9, 1771–1791. [Google Scholar] [CrossRef] [Green Version]
- Ruggeri, F.; Marcott, C.; Dinarelli, S.; Longo, G.; Girasole, M.; Dietler, G.; Knowles, T. Identification of Oxidative Stress in Red Blood Cells with Nanoscale Chemical Resolution by Infrared Nanospectroscopy. Int. J. Mol. Sci. 2018, 19, 2582. [Google Scholar] [CrossRef] [Green Version]
- Saladrigas-Manjón, S.; Dučić, T.; Galindo, L.; Fernández-Avilés, C.; Pérez, V.; de la Torre, R.; Robledo, P. Effects of Cannabis Use on the Protein and Lipid Profile of Olfactory Neuroepithelium Cells from Schizophrenia Patients Studied by Synchrotron-Based FTIR Spectroscopy. Biomolecules 2020, 10, 329. [Google Scholar] [CrossRef] [Green Version]
- Sánchez, V.; Redmann, K.; Wistuba, J.; Wübbeling, F.; Burger, M.; Oldenhof, H.; Wolkers, W.F.; Kliesch, S.; Schlatt, S.; Mallidis, C. Oxidative DNA damage in human sperm can be detected by Raman microspectroscopy. Fertil. Steril. 2012, 98, 1124–1129.e3. [Google Scholar] [CrossRef]
- Fodor, S.P.A.; Spiro, T.G. Ultraviolet resonance Raman spectroscopy of DNA with 200-266-nm laser excitation. J. Am. Chem. Soc. 1986, 108, 3198–3205. [Google Scholar] [CrossRef]
- Zhu, J.; Barratt, C.L.R.; Lippes, J.; Pacey, A.A.; Lenton, E.A.; Cooke, I.D. Human oviductal fluid prolongs sperm survival. Fertil. Steril. 1994, 61, 360–366. [Google Scholar] [CrossRef]
- Aitken, R.J.; Nixon, B. Sperm capacitation: A distant landscape glimpsed but unexplored. Mol. Hum. Reprod. 2013, 19, 785–793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Lamirande, E. Capacitation as a regulatory event that primes spermatozoa for the acrosome reaction and fertilization. Mol. Hum. Reprod. 1997, 3, 175–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langlais, J.; Roberts, K.D. A molecular membrane model of sperm capacitation and the acrosome reaction of mammalian spermatozoa. Gamete Res. 1985, 12, 183–224. [Google Scholar] [CrossRef]
- Cheng, P.-N.; Pham, J.D.; Nowick, J.S. The Supramolecular Chemistry of β-Sheets. J. Am. Chem. Soc. 2013, 135, 5477–5492. [Google Scholar] [CrossRef] [Green Version]
- Baker, M.A.; Nixon, B.; Naumovski, N.; Aitken, R.J. Proteomic insights into the maturation and capacitation of mammalian spermatozoa. Syst. Biol. Reprod. Med. 2012, 58, 211–217. [Google Scholar] [CrossRef]
- Walters, J.L.H.; Gadella, B.M.; Sutherland, J.M.; Nixon, B.; Bromfield, E.G. Male Infertility: Shining a Light on Lipids and Lipid-Modulating Enzymes in the Male Germline. J. Clin. Med. 2020, 9, 327. [Google Scholar] [CrossRef] [Green Version]
- Flesch, F.M.; Gadella, B.M. Dynamics of the mammalian sperm plasma membrane in the process of fertilization. Biochim. Biophys. Acta (BBA)-Rev. Biomembr. 2000, 1469, 197–235. [Google Scholar] [CrossRef]
- Lenzi, A. Lipids of the sperm plasma membrane: From polyunsaturated fatty acids considered as markers of sperm function to possible scavenger therapy. Hum. Reprod. Update 1996, 2, 246–256. [Google Scholar] [CrossRef] [Green Version]
- Lenzi, A.; Gandini, L.; Maresca, V.; Rago, R.; Sgro, P.; Dondero, F.; Picardo, M. Fatty acid composition of spermatozoa and immature germ cells. Mol. Hum. Reprod. 2000, 6, 226–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aksoy, Y.; Aksoy, H.; Altınkaynak, K.; Aydın, H.R.; Özkan, A. Sperm fatty acid composition in subfertile men. Prostaglandins Leukot. Essent. Fat. Acids 2006, 75, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Zalata, A. The fatty acid composition of phospholipids of spermatozoa from infertile patients. Mol. Hum. Reprod. 1998, 4, 111–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zerbinati, C.; Caponecchia, L.; Rago, R.; Leoncini, E.; Bottaccioli, A.G.; Ciacciarelli, M.; Pacelli, A.; Salacone, P.; Sebastianelli, A.; Pastore, A.; et al. Fatty acids profiling reveals potential candidate markers of semen quality. Andrology 2016, 4, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- Gulaya, N.M.; Margitich, V.M.; Govseeva, N.M.; Klimashevsky, V.M.; Gorpynchenko, I.I.; Boyko, M.I. Phospholipid composition of human sperm and seminal plasma in relation to sperm fertility. Arch. Androl. 2001, 46, 169–175. [Google Scholar] [CrossRef] [Green Version]
- Connor, W.E.; Weleber, R.G.; DeFrancesco, C.; Lin, D.S.; Wolf, D.P. Sperm abnormalities in retinitis pigmentosa. Investig. Ophthalmol. Vis. Sci. 1997, 38, 2619–2628. [Google Scholar]
- Kahn, B.E.; Brannigan, R.E. Obesity and male infertility. Curr. Opin. Urol. 2017, 27, 441–445. [Google Scholar] [CrossRef]
- Chen, S.; Allam, J.-P.; Duan, Y.; Haidl, G. Influence of reactive oxygen species on human sperm functions and fertilizing capacity including therapeutical approaches. Arch. Gynecol. Obstet. 2013, 288, 191–199. [Google Scholar] [CrossRef]
- Balan, V.; Mihai, C.-T.; Cojocaru, F.-D.; Uritu, C.-M.; Dodi, G.; Botezat, D.; Gardikiotis, I. Vibrational Spectroscopy Fingerprinting in Medicine: From Molecular to Clinical Practice. Materials 2019, 12, 2884. [Google Scholar] [CrossRef] [Green Version]







| Motility (%) | |||
|---|---|---|---|
| Fraction 1 | Fraction 3 | Total | |
| Patient 1 | 84 | 44 | 53 |
| Patient 2 | 27 | 11 | 27 |
| Patient 3 | 97 | 20 | 55 |
| Patient 4 | 87 | 61 | 64 |
| Patient 5 | 46 | 25 | 41 |
| Patient 6 | 54 | 22 | 27 |
| Assignment | Peak Position (cm−1) | Literature |
|---|---|---|
| β-sheets (intermolecular, extended) | 1615–1628 | [19,21,22,23] |
| β-sheets (intermolecular, short) | 1629–1635 1680–1696 | [19,21,22,23,24] |
| α-helix | 1650–1656 | [19,25] |
| β-turn/loops | 1670–1678 | [19,25] |
| Assignment | Peak Position (cm−1) | Literature |
|---|---|---|
| CH2 symmetric stretching | 2850–2854 | [14,26,27] |
| CH3 symmetric stretching | 2870 | [14,26,27] |
| CH2 asymmetric stretching | 2925 | [14,27,28] |
| CH3 asymmetric stretching | 2956 | [14,26,27] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pachetti, M.; Zupin, L.; Venturin, I.; Mitri, E.; Boscolo, R.; D’Amico, F.; Vaccari, L.; Crovella, S.; Ricci, G.; Pascolo, L. FTIR Spectroscopy to Reveal Lipid and Protein Changes Induced on Sperm by Capacitation: Bases for an Improvement of Sample Selection in ART. Int. J. Mol. Sci. 2020, 21, 8659. https://doi.org/10.3390/ijms21228659
Pachetti M, Zupin L, Venturin I, Mitri E, Boscolo R, D’Amico F, Vaccari L, Crovella S, Ricci G, Pascolo L. FTIR Spectroscopy to Reveal Lipid and Protein Changes Induced on Sperm by Capacitation: Bases for an Improvement of Sample Selection in ART. International Journal of Molecular Sciences. 2020; 21(22):8659. https://doi.org/10.3390/ijms21228659
Chicago/Turabian StylePachetti, Maria, Luisa Zupin, Irene Venturin, Elisa Mitri, Rita Boscolo, Francesco D’Amico, Lisa Vaccari, Sergio Crovella, Giuseppe Ricci, and Lorella Pascolo. 2020. "FTIR Spectroscopy to Reveal Lipid and Protein Changes Induced on Sperm by Capacitation: Bases for an Improvement of Sample Selection in ART" International Journal of Molecular Sciences 21, no. 22: 8659. https://doi.org/10.3390/ijms21228659
APA StylePachetti, M., Zupin, L., Venturin, I., Mitri, E., Boscolo, R., D’Amico, F., Vaccari, L., Crovella, S., Ricci, G., & Pascolo, L. (2020). FTIR Spectroscopy to Reveal Lipid and Protein Changes Induced on Sperm by Capacitation: Bases for an Improvement of Sample Selection in ART. International Journal of Molecular Sciences, 21(22), 8659. https://doi.org/10.3390/ijms21228659