Ovarian Aging: Molecular Mechanisms and Medical Management
Abstract
:1. Introduction
2. Methods
3. Molecular Mechanisms
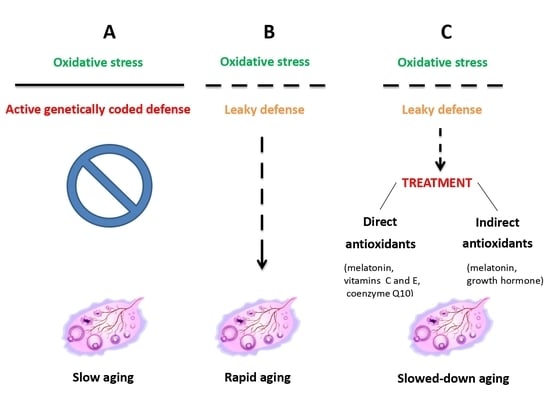
3.1. Genetic Basis
3.1.1. Primary Ovarian Insufficiency
3.1.2. Ovarian Insufficiency due to Mendelian Disorders Implicated in Other Pathologies
3.1.3. Gene Mutations Affecting Mitochondrial Function
3.2. Cell-Signaling Pathways
4. Clinical Management
4.1. Diagnosis
4.2. Treatment
4.2.1. GH
4.2.2. Melatonin
4.2.3. Other Antioxidants
4.2.4. Mitochondrial Therapy
4.2.5. Patient-Tailored (Customized) Treatment Protocols
5. Conclusions
Funding
Conflicts of Interest
References
- Dviri, M.; Madjunkova, S.; Koziarz, A.; Antes, R.; Abramov, R.; Mashiach, J.; Moskovtsev, S.; Kuznyetsova, I.; Librach, C. Is there a correlation between paternal age and aneuploidy rate? An analysis of 3,118 embryos derived from young egg donors. Fertil. Steril. 2020, 114, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Franasiak, J.M.; Forman, E.J.; Hong, K.H.; Werner, M.D.; Upham, K.M.; Treff, N.R.; Scott, R.T., Jr. The nature of aneuploidy with increasing age of the female partner: A review of 15,169 consecutive trophectoderm biopsies evaluated with comprehensive chromosomal screening. Fertil. Steril. 2014, 101, 656–663.e1. [Google Scholar] [CrossRef] [PubMed]
- Demko, Z.P.; Simon, A.L.; McCoy, R.C.; Petrov, D.A.; Rabinowitz, M. Effects of maternal age on euploidy rates in a large cohort of embryos analyzed with 24-chromosome single-nucleotide polymorphism-based preimplantation genetic screening. Fertil. Steril. 2016, 105, 1307–1313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossetti, R.; Ferrari, I.; Bonomi, M.; Persani, L. Genetics of primary ovarian insufficiency. Clin. Genet. 2017, 91, 183–198. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Lu, H.; Chen, R.; Wu, M.; Jin, Y.; Zhang, J.; Wang, S. Identification of key genes and potential new biomarkers for ovarian aging: A study based on RNA-sequencing data. Front. Genet. 2020, 11, 590660. [Google Scholar] [CrossRef]
- Turan, V.; Oktay, K. BRCA-related ATM-mediated DNA double-strand break repair and ovarian aging. Hum. Reprod. Update 2020, 26, 43–57. [Google Scholar] [CrossRef]
- te Velde, E.R.; Pearson, P.L. The variability of female reproductive ageing. Hum. Reprod. Update 2002, 8, 141–154. [Google Scholar] [CrossRef]
- Qin, Y.; Jiao, X.; Simpson, J.L.; Chen, Z.J. Genetics of primary ovarian insufficiency: New developments and opportunities. Hum. Reprod. Update 2015, 21, 787–808. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, Y.; Li, J.; Yu, Y.; Zhang, W.; Song, M.; Liu, Z.; Min, Z.; Hu, H.; Jing, Y.; et al. Single-cell transcriptomic atlas of primate ovarian aging. Cell 2020, 180, 585–600.e19. [Google Scholar] [CrossRef]
- Jin, M.; Yu, Y.; Huang, H. An update on primary ovarian insufficiency. Sci. China Life Sci. 2012, 55, 677–686. [Google Scholar] [CrossRef] [Green Version]
- Fortuño, C.; Labarta, E. Genetics of primary ovarian insufficiency: A review. J. Assist. Reprod. Genet. 2014, 31, 1573–1585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luoma, P.; Melberg, A.; Rinne, J.O.; Kaukonen, J.A.; Nupponen, N.N.; Chalmers, R.M.; Oldfors, A.; Rautakorpi, I.; Peltonen, L.; Majamaa, K.; et al. Parkinsonism, premature menopause, and mitochondrial DNA polymerase gamma mutations: Clinical and molecular genetic study. Lancet 2004, 364, 875–882. [Google Scholar] [CrossRef]
- Pierce, S.B.; Gersak, K.; Michaelson-Cohen, R.; Walsh, T.; Lee, M.K.; Malach, D.; Klevit, R.E.; King, M.C.; Levy-Lahad, E. Mutations in LARS2, encoding mitochondrial leucyl-tRNA synthetase, lead to premature ovarian failure and hearing loss in Perrault syndrome. Am. J. Hum. Genet. 2013, 92, 614–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, J.; Luderer, U. Oxidative damage increases and antioxidant gene expression decreases with aging in the mouse ovary. Biol. Reprod. 2011, 84, 775–782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoque, S.A.M.; Kawai, T.; Zhu, Z.; Shimada, M. Mitochondrial protein turnover is critical for granulosa cell proliferation and differentiation in antral follicles. J. Endocr. Soc. 2019, 3, 324–339. [Google Scholar] [CrossRef] [Green Version]
- May-Panloup, P.; Boucret, L.; Chao de la Barca, J.-M.; Desquiret-Dumas, V.; Ferré-L’Hotellier, V.; Morinière, C.; Descamps, P.; Procaccio, V.; Reynier, P. Ovarian ageing: The role of mitochondria in oocytes and follicles. Hum. Reprod. Update 2016, 22, 725–743. [Google Scholar] [CrossRef] [Green Version]
- Mikhailova, A.A.; Shamanskyi, V.; Ushakova, K.; Mikhailova, A.G.; Oreshkov, S.; Knorre, D.; Tretiakov, E.O.; Zazhytska, M.; Lukowski, S.W.; Liou, C.-W.; et al. Risk of mitochondrial deletions is affected by the global secondary structure of the mitochondrial genome. bioRxiv 2020, 603282. [Google Scholar] [CrossRef] [Green Version]
- Pizarro, B.M.; Cordeiro, A.; Reginatto, M.W.; Campos, S.P.C.; Mancebo, A.C.A.; Areas, P.C.F.; Antunes, R.A.; Souza, M.D.C.B.; Oliveira, K.J.; Bloise, F.F.; et al. Estradiol and progesterone levels are related to redox status in the follicular fluid during in vitro fertilization. J. Endocr. Soc. 2020, 4, bvaa064. [Google Scholar] [CrossRef]
- Tesarik, J.; Mendoza, C. Nongenomic effects of 17 beta-estradiol on maturing human oocytes: Relationship to oocyte developmental potential. J. Clin. Endocrinol. Metab. 1995, 80, 1438–1443. [Google Scholar] [CrossRef] [Green Version]
- Tang, R.; Yu, Q. Novel variants in women with premature ovarian function decline identified via whole-exome sequencing. J. Assist. Reprod. Genet. 2020, 37, 2487–2502. [Google Scholar] [CrossRef]
- Barros, F.; Carvalho, F.; Barros, A.; Dória, S. Premature ovarian insufficiency: Clinical orientations for genetic testing and genetic counseling. Porto Biomed. J. 2020, 5, e62. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.L.; Stevens, A.; Minogue, B.; Sneddon, S.; Shaw, L.; Wood, L.; Adeniyi, T.; Xiao, H.; Lio, P.; Kimber, S.J.; et al. Systems based analysis of human embryos and gene networks involved in cell lineage allocation. BMC Genom. 2019, 20, 171. [Google Scholar] [CrossRef] [PubMed]
- Fleming, R.; Seifer, D.B.; Frattarelli, J.L.; Ruman, J. Assessing ovarian response: Antral follicle count versus anti-Müllerian hormone. Reprod. Biomed. Online 2015, 31, 486–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yovich, J.L.; Regan, S.L.P.; Zaidi, S.; Keane, K.N. The concept of growth hormone deficiency affecting clinical prognosis in IVF. Front. Endocrinol. 2019, 10, 650. [Google Scholar] [CrossRef] [PubMed]
- Tesarik, J.; Mendoza-Tesarik, R. New criteria for the use of growth hormone in the treatment of female infertility: Minireview and a case series. EC Gynaecol. 2020, 9, 1–4. [Google Scholar]
- Tesarik, J.; Hazout, A.; Mendoza, C. Improvement of delivery and live birth rates after ICSI in women aged >40 years by ovarian co-stimulation with growth hormone. Hum. Reprod. 2005, 20, 2536–2541. [Google Scholar] [CrossRef] [Green Version]
- Li, X.L.; Wang, L.; Lv, F.; Huang, X.M.; Wang, L.P.; Pan, Y.; Zhang, X.M. The influence of different growth hormone addition protocols to poor ovarian responders on clinical outcomes in controlled ovary stimulation cycles: A systematic review and meta-analysis. Medicine 2017, 96, e6443. [Google Scholar] [CrossRef]
- Hart, R.J. Use of growth hormone in the IVF treatment of women with poor ovarian reserve. Front. Endocrinol. 2019, 10, 500. [Google Scholar] [CrossRef] [Green Version]
- Tesarik, J.; Galán-Lázaro, M.; Conde-López, C.; Chiara-Rapisarda, A.M.; Mendoza-Tesarik, R. The effect of GH administration on oocyte and zygote quality in young women with repeated implantation failure after IVF. Front. Endocrinol. 2020, 11, 519572. [Google Scholar] [CrossRef]
- Gong, Y.; Luo, S.; Fan, P.; Jin, S.; Zhu, H.; Deng, T.; Quan, Y.; Huang, W. Growth hormone alleviates oxidative stress and improves oocyte quality in Chinese women with polycystic ovary syndrome: A randomized controlled trial. Sci. Rep. 2020, 11, 18769. [Google Scholar] [CrossRef]
- Mancini, A.; Di Segni, C.; Bruno, C.; Olivieri, G.; Guidi, F.; Silvestrini, A.; Meucci, E.; Orlando, P.; Silvestri, S.; Tiano, L.; et al. Oxidative stress in adult growth hormone deficiency: Different plasma antioxidant patterns in comparison with metabolic syndrome. Endocrine 2018, 59, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Tesarik, J.; Yovich, J.; Menezo, Y. Growth Hormone in Fertility and Infertility: Physiology, Pathology, Diagnosis and Treatment. Front. Endocrinol. 2020, in press. [Google Scholar]
- Tesarik, J.; Mendoza-Tesarik, R. Melatonin: The first noninvasive causal therapy for both endometriosis and adenomyosis? J. Gynecol. Women’s Health 2018, 12, 555829. [Google Scholar] [CrossRef]
- Mosher, A.A.; Tsoulis, M.W.; Lim, J.; Tan, C.; Agarwal, S.K.; Leyland, N.A.; Foster, W.G. Melatonin activity and receptor expression in endometrial tissue and endometriosis. Hum. Reprod. 2019, 34, 1215–1224. [Google Scholar] [CrossRef]
- Tesarik, J. Melatonin attenuates growth factor receptor signaling required for SARS-CoV-2 replication. Melatonin Res. 2020, 3, 534–537. [Google Scholar] [CrossRef]
- Tamura, H.; Jozaki, M.; Tanabe, M.; Shirafuta, Y.; Mihara, Y.; Shinagawa, M.; Tamura, I.; Maekawa, R.; Sato, S.; Taketani, T.; et al. Importance of melatonin in assisted reproductive technology and ovarian aging. Int. J. Mol. Sci. 2020, 21, 1135. [Google Scholar] [CrossRef] [Green Version]
- Budani, M.C.; Tiboni, G.M. Effects of Supplementation with Natural Antioxidants on Oocytes and Preimplantation Embryos. Antioxidants 2020, 9, 612. [Google Scholar] [CrossRef]
- Gat, I.; Blanco Mejia, S.; Balakier, H.; Librach, C.L.; Claessens, A.; Ryan, E.A. The use of coenzyme Q10 and DHEA during IUI and IVF cycles in patients with decreased ovarian reserve. Gynecol. Endocrinol. 2016, 32, 534–537. [Google Scholar] [CrossRef]
- Xu, Y.; Nisenblat, V.; Lu, C.; Li, R.; Qiao, J.; Zhen, X.; Wang, S. Pretreatment with coenzyme Q10 improves ovarian response and embryo quality in low-prognosis young women with decreased ovarian reserve: A randomized controlled trial. Reprod. Biol. Endocrinol. 2018, 16, 29. [Google Scholar] [CrossRef]
- Agarwal, A.; Durairajanayagam, D.; du Plessis, S.S. Utility of antioxidants during assisted reproductive techniques: An evidence based review. Reprod. Biol. Endocrinol. 2014, 12, 112. [Google Scholar] [CrossRef] [Green Version]
- Babayev, E.; Seli, E. Oocyte mitochondrial function and reproduction. Curr. Opin. Obstet. Gynecol. 2015, 27, 175–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tachibana, M.; Kuno, T.; Yaegashi, N. Mitochondrial replacement therapy and assisted reproductive technology: A paradigm shift toward treatment of genetic diseases in gametes or in early embryos. Reprod. Med. Biol. 2018, 17, 421–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, J.; Scott, R.; Schimmel, T.; Levron, J.; Willadsen, S. Birth of infant after transfer of anucleate donor oocyte cytoplasm into recipient eggs. Lancet 1997, 350, 186–187. [Google Scholar] [CrossRef]
- Cohen, J.; Scott, R.; Alikani, M.; Schimmel, T.; Munné, S.; Levron, J.; Wu, L.; Brenner, C.; Warner, C.; Willadsen, S. Ooplasmic transfer in mature human oocytes. Mol. Hum. Reprod. 1998, 4, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Tesarik, J.; Nagy, Z.P.; Mendoza, C.; Greco, E. Chemically and mechanically induced membrane fusion: Non-activating methods for nuclear transfer in mature human oocytes. Hum. Reprod. 2000, 15, 1149–1154. [Google Scholar] [CrossRef] [Green Version]
- Tesarik, J. Forty years of in vitro fertilisation: A history of continuous expansion. In 40 Years after In Vitro Fertilisation: State of the Art and New Challenges; Tesarik, J., Ed.; Cambridge Scholars Publishing: Newcastle upon Tyne, UK, 2019; pp. 1–24. [Google Scholar]
- Malter, H.E. Improving oocytes and embryos? Cytoplasmic manipulation in human reproduction. In 40 Years after In Vitro Fertilisation: State of the Art and New Challenges; Tesarik, J., Ed.; Cambridge Scholars Publishing: Newcastle upon Tyne, UK, 2019; pp. 234–268. [Google Scholar]
- Tesarik, J.; Kopecný, V.; Plachot, M.; Mandelbaum, J. Activation of nucleolar and extranucleolar RNA synthesis and changes in the ribosomal content of human embryos developing in vitro. J. Reprod. Fertil. 1986, 78, 463–470. [Google Scholar] [CrossRef] [Green Version]
- Braude, P.; Bolton, V.; Moore, S. Human gene expression first occurs between the four- and eight-cell stages of preimplantation development. Nature 1988, 332, 459–461. [Google Scholar] [CrossRef]
- Tesarik, J.; Kopecný, V.; Plachot, M.; Mandelbaum, J. Early morphological signs of embryonic genome expression in human preimplantation development as revealed by quantitative electron microscopy. Dev. Biol. 1988, 128, 15–20. [Google Scholar] [CrossRef]
- Tesarik, J. Involvement of oocyte-coded message in cell differentiation control of early human embryos. Development 1989, 105, 317–322. [Google Scholar]
- Zhang, J.; Liu, H.; Luo, S.; Lu, Z.; Chávez-Badiola, A.; Liu, Z.; Yang, M.; Merhi, Z.; Silber, S.J.; Munné, S.; et al. Live birth derived from oocyte spindle transfer to prevent mitochondrial disease. Reprod. Biomed. Online 2017, 34, 361–368. [Google Scholar] [CrossRef] [Green Version]
- Tesarik, J. Oocyte spindle transfer for prevention of mitochondrial disease: The question of membrane fusion technique. Reprod. Biomed. Online 2017, 35, 432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tesarik, J. Customized assisted reproduction enhancement (CARE) for women with extremely poor ovarian reserve (EPOR). J. Gynecol. Women Health 2017, 3, 555625. [Google Scholar] [CrossRef] [Green Version]
- Mendoza-Tesarik, R.; Tesarik, J. Usefulness of individualized FSH, LH and GH dosing in ovarian stimulation of women with low ovarian reserve. Hum. Reprod. 2018, 33, 981–982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Gene | Location | Function |
|---|---|---|
| WNT4 | 1p36.23-p35.1 | Female sex determination and differentiation |
| FIGLA | 2p13.3 | Primordial follicle and zona pellucida formation |
| NOBOX | 7q35 | Transition from primordial to growing follicles |
| FOXO3 | 6q21 | Transition from primordial to growing follicles |
| PTEN | 10q23.3 | Transition from primordial to growing follicles |
| FSHR | 2p21-p16 | Hormone-dependent phase of follicular growth |
| GPR3 | 1p36.1-p35 | Maintenance of meiotic arrest until the LH surge |
| MSH4 | 1p31 | DNA mismatch repair during meiotic recombination |
| MSH5 | 6p21.3 | DNA mismatch repair during meiotic recombination |
| PGRMC1 | Xq22-q24 | Apoptosis of ovarian cells |
| FOXO1 | 13q14.1 | Granulosa cell function |
| DMC1 | 22q13.1 | Repair of DNA damage during meiotic divisions |
| Agent | Administration | Mechanisms of Action | References |
|---|---|---|---|
| GH | Subcutaneous | Activation of cell-signaling pathways acting | [26,27,28,29,30,31,32] |
| against oxidative stress | |||
| Possible activation of DNA damage repair | |||
| Melatonin | Oral | Direct antioxidant | [33,34,35,36] |
| Indirect antioxidant (signaling pathway modulator) | |||
| Anti-inflammatory agent | |||
| Immunomodulator | |||
| Coenzyme Q10 | Oral | Direct antioxidant | [37,38,39] |
| Vitamin C | Oral | Direct antioxidant | [40] |
| Vitamin E | Oral | Direct antioxidant | [40] |
| Folic acid | Oral | Direct antioxidant | [40] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tesarik, J.; Galán-Lázaro, M.; Mendoza-Tesarik, R. Ovarian Aging: Molecular Mechanisms and Medical Management. Int. J. Mol. Sci. 2021, 22, 1371. https://doi.org/10.3390/ijms22031371
Tesarik J, Galán-Lázaro M, Mendoza-Tesarik R. Ovarian Aging: Molecular Mechanisms and Medical Management. International Journal of Molecular Sciences. 2021; 22(3):1371. https://doi.org/10.3390/ijms22031371
Chicago/Turabian StyleTesarik, Jan, Maribel Galán-Lázaro, and Raquel Mendoza-Tesarik. 2021. "Ovarian Aging: Molecular Mechanisms and Medical Management" International Journal of Molecular Sciences 22, no. 3: 1371. https://doi.org/10.3390/ijms22031371
APA StyleTesarik, J., Galán-Lázaro, M., & Mendoza-Tesarik, R. (2021). Ovarian Aging: Molecular Mechanisms and Medical Management. International Journal of Molecular Sciences, 22(3), 1371. https://doi.org/10.3390/ijms22031371