On the Single-Crystal Structure of Tenofovir Alafenamide Mono-Fumarate: A Metastable Phase Featuring a Mixture of Co-Crystal and Salt
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Spectroscopic Characterizations
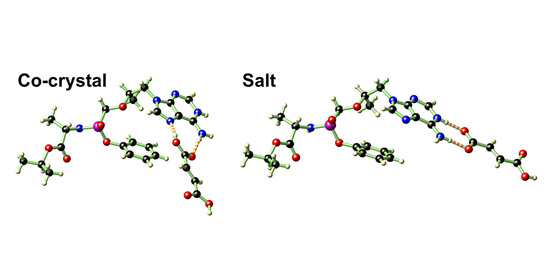
2.2. Single-Crystal Structure Analysis of 2
2.3. Hirshfeld Surface Analysis of TAF, 1, and 2
3. Materials and Methods
3.1. General
3.2. Crystallization of TAF Fumarate and Its Characterization Data
3.3. X-ray Crystallography
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Abdool Karim, Q.; Abdool Karim, S.S.; Frohlich, J.A.; Grobler, A.C.; Baxter, C.; Mansoor, L.E.; Kharsany, A.B.M.; Sibeko, S.; Mlisana, K.P.; Omar, Z.; et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science 2010, 329, 1168–1174. [Google Scholar] [CrossRef] [Green Version]
- De Clercq, E. Anti-HIV drugs: 25 compounds approved within 25 years after the discovery of HIV. Int. J. Antimicrob. Agents 2009, 33, 307–320. [Google Scholar] [CrossRef]
- Mahapatra, S.; Thakur, T.S.; Joseph, S.; Varughese, S.; Desiraju, G.R. New solid state forms of the anti-hiv drug efavirenz. Conformational flexibility and high Z’ issues. Cryst. Growth Des. 2010, 10, 3191–3202. [Google Scholar] [CrossRef]
- Callebaut, C.; Stepan, G.; Tian, Y.; Miller, M.D. In vitro virology profile of tenofovir alafenamide, a novel oral prodrug of tenofovir with improved antiviral activity compared to that of tenofovir disoproxil fumarate. Antimicrob. Agents Chemother. 2015, 59, 5909–5916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, F.-L.; Yang, S.-P.; Zhang, W.-H.; Liu, Q.; Yu, H.; Chen, J.-X.; Lang, J.-P. Counterintuitive solid-state syntheses of indium-thiolate-phen cations as efficient and selective fluorescent biosensors for HIV-1 ds-DNA and Sudan Ebolavirus RNA sequences. ChemistrySelect 2016, 1, 2979–2987. [Google Scholar] [CrossRef]
- Zhao, H.-Q.; Yang, S.-P.; Ding, N.-N.; Qin, L.; Qiu, G.-H.; Chen, J.-X.; Zhang, W.-H.; Chen, W.-H.; Hor, T.S.A. A zwitterionic 1D/2D polymer co-crystal and its polymorphic sub-components: A highly selective sensing platform for HIV ds-DNA sequences. Dalton Trans. 2016, 45, 5092–5100. [Google Scholar] [CrossRef]
- Lesbats, P.; Engelman, A.N.; Cherepanov, P. Retroviral DNA integration. Chem. Rev. 2016, 116, 12730–12757. [Google Scholar] [CrossRef] [Green Version]
- Rezaei, T.; Khalili, S.; Baradaran, B.; Mosafer, J.; Rezaei, S.; Mokhtarzadeh, A.; de la Guardia, M. Recent advances on HIV DNA vaccines development: Stepwise improvements to clinical trials. J. Control. Release 2019, 316, 116–137. [Google Scholar] [CrossRef]
- Liaw, Y.F. Hepatitis B virus replication and liver disease progression: The impact of antiviral therapy. Antivir. Ther. 2006, 11, 669–679. [Google Scholar]
- Cooper, R.D.; Wiebe, N.; Smith, N.; Keiser, P.; Naicker, S.; Tonelli, M. Systematic review and meta-analysis: Renal safety of tenofovir disoproxil fumarate in HIV-infected patients. Clin. Infect. Dis. 2010, 51, 496–505. [Google Scholar] [CrossRef] [Green Version]
- Sax, P.E.; Wohl, D.; Yin, M.T.; Post, F.; DeJesus, E.; Saag, M.; Pozniak, A.; Thompson, M.; Podzamczer, D.; Molina, J.M.; et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate, coformulated with elvitegravir, cobicistat, and emtricitabine, for initial treatment of HIV-1 infection: Two randomised, double-blind, phase 3, non-inferiority trials. Lancet 2015, 385, 2606–2615. [Google Scholar] [CrossRef]
- Ray, A.S.; Fordyce, M.W.; Hitchcock, M.J.M. Tenofovir alafenamide: A novel prodrug of tenofovir for the treatment of Human Immunodeficiency Virus. Antivir. Res. 2016, 125, 63–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lengauer, H.; Makuc, D.; Šterk, D.; Perdih, F.; Pichler, A.; Trdan Lušin, T.; Plavec, J.; Časar, Z. Co-crystals, salts or mixtures of both? The case of tenofovir alafenamide fumarates. Pharmaceutics 2020, 12, 342. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lu, X.; Yang, X.; Xu, N. The efficacy and safety of tenofovir alafenamide versus tenofovir disoproxil fumarate in antiretroviral regimens for HIV-1 therapy: Meta-analysis. Medicine 2016, 95, e5146. [Google Scholar] [CrossRef]
- Meng, X. Crystal Form of Tenofovir Alafenamide Salt, Preparation Method and Use Thereof. U.S. Patent Application No. 10479810, 19 November 2019. [Google Scholar]
- Han, L.; Hu, W.; Wang, H. Preparing Tenofovir Alafenamide Hemifumarate, Comprises e.g. Reacting Tenofovir and Triphenyl Phosphate, Reacting with Acylating Agent, Amidating with L-alanine Isopropyl Ester, Inducing and Salt Forming with Fumaric Acid in Solvent. CN110305163-A, 8 October 2019. [Google Scholar]
- Hahn, J.; Park, Y.K.; Park, S.; Kim, C.; Shin, S.; Choi, W.S.; Gi, K. New Tenofovir Alafenamide Succinate Salt with Endothermic Peak Measured by Differential Scanning Calorimetry, Useful in Pharmaceutical Composition. KR2020084713-A, 3 January 2019. [Google Scholar]
- Gotham, D.; Hill, A.; Pozniak, A.L. Candidates for inclusion in a universal antiretroviral regimen: Tenofovir alafenamide. Curr. Opin. HIV AIDS 2017, 12, 324–333. [Google Scholar] [CrossRef]
- Kim, Y.S.; Oka, S.; Chetchotisakd, P.; Clarke, A.; Supparatpinyo, K.; Avihingsanon, A.; Ratanasuwan, W.; Kiertiburanakul, S.; Ruxrungtham, K.; Yang, S.; et al. Efficacy and safety of elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide in Asian participants with human immunodeficiency virus 1 infection: A sub-analysis of phase 3 clinical trials. HIV Res. Clin. Pract. 2019, 1–9. [Google Scholar] [CrossRef]
- Viganò, M.; Loglio, A.; Grossi, G.; Lampertico, P. Tenofovir alafenamide (TAF) treatment of HBV, what are the unanswered questions? Expert Rev. Anti. Infect. Ther. 2018, 16, 153–161. [Google Scholar] [CrossRef]
- Becker, M.W.; Chapman, H.H.; Cihlar, T.; Eisenberg, E.J.; He, G.-X.; Kernan, M.R.; Lee, W.A.; Prisbe, E.J.; Rohloff, J.C.; Sparacino, M.L. Prodrugs of Phosphonate Nucleotide Analogues and Methods for Selecting and Making Same. U.S. Patent Application No. 20020119443 A1, 29 August 2002. [Google Scholar]
- Childs, S.L.; Stahly, G.P.; Park, A. The salt–cocrystal continuum: The influence of crystal structure on ionization state. Mol. Pharm. 2007, 4, 323–338. [Google Scholar] [CrossRef] [Green Version]
- Papageorgiou, S.K.; Kouvelos, E.P.; Favvas, E.P.; Sapalidis, A.A.; Romanos, G.E.; Katsaros, F.K. Metal–carboxylate interactions in metal–alginate complexes studied with FTIR spectroscopy. Carbohydr. Res. 2010, 345, 469–473. [Google Scholar] [CrossRef]
- Mary, Y.S.; Ushakumari, L.; Harikumar, B.; Varghese, H.T.; Panicker, C.Y. FT-IR, FT-raman and SERS spectra of L-proline. J. Iran. Chem. Soc. 2009, 6, 138–144. [Google Scholar] [CrossRef]
- Yuan, F.-L.; Yuan, Y.-Q.; Chao, M.-Y.; Young, D.J.; Zhang, W.-H.; Lang, J.-P. Deciphering the structural relationships of five Cd-based metal–organic frameworks. Inorg. Chem. 2017, 56, 6522–6531. [Google Scholar] [CrossRef] [PubMed]
- Armaghan, M.; Lu, W.Y.J.; Wu, D.; Wei, Y.; Yuan, F.-L.; Ng, S.W.; Amini, M.M.; Zhang, W.-H.; Young, D.J.; Hor, T.S.A.; et al. Isolation of first row transition metal-carboxylate zwitterions. RSC Adv. 2015, 5, 42978–42989. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Lin, S.-X.; Niu, R.-J.; Liu, Q.; Zhang, W.-H.; Young, D.J. Zinc and cadmium complexes of pyridinemethanol carboxylates: Metal carboxylate zwitterions and metal–organic frameworks. ChemPlusChem 2020, 85, 832–837. [Google Scholar] [CrossRef] [PubMed]
- Armaghan, M.; Shang, X.J.; Yuan, Y.Q.; Young, D.J.; Zhang, W.H.; Hor, T.S.A.; Lang, J.P. Metal–organic frameworks via emissive metal-carboxylate zwitterion intermediates. ChemPlusChem 2015, 80, 1231–1234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Wolff, S.; Grimwood, D.; McKinnon, J.; Turner, M.; Jayatilaka, D.; Spackman, M. Crystal Explorer; The University of Western Australia: Crawley, Australia, 2012. [Google Scholar]
- Higashi, T. ABSCOR; Rigaku Corporation: Tokyo, Japan, 1995. [Google Scholar]
- Sheldrick, G. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX suite for smallmolecule single-crystal crystallography. J. Appl. Cryst. 1999, 32, 837–838. [Google Scholar] [CrossRef]









| Empirical Formula | C50H68N12O19P2 |
|---|---|
| Temperature | 296 (2) K |
| Formula weight | 1203.10 |
| Crystal system | monoclinic |
| Space group | P21 |
| a/Å | 9.7367 (3) |
| b/Å | 18.1659 (5) |
| c/Å | 16.7114 (5) |
| α/° | 90 |
| β/° | 98.673 (3) |
| γ/° | 90 |
| V/Å3 | 2922.04 (15) |
| Z | 2 |
| Dc/(g cm−3) | 1.367 |
| μ (Mo-Kα)/mm−1 | 0.157 |
| F(000) | 1268 |
| Total reflections | 69,895 |
| Unique reflections | 11,469 |
| No observations | 9900 |
| No parameters | 803 |
| Flack parameter | 0.00 (4) |
| Rint | 0.0586 |
| R1 | 0.0430 |
| wR2 | 0.0985 |
| GOF3 | 1.040 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.-W.; Liu, L.; Yu, K.-X.; Bai, H.-Z.; Zhou, J.; Zhang, W.-H.; Hu, X.; Tang, G. On the Single-Crystal Structure of Tenofovir Alafenamide Mono-Fumarate: A Metastable Phase Featuring a Mixture of Co-Crystal and Salt. Int. J. Mol. Sci. 2020, 21, 9213. https://doi.org/10.3390/ijms21239213
Wang J-W, Liu L, Yu K-X, Bai H-Z, Zhou J, Zhang W-H, Hu X, Tang G. On the Single-Crystal Structure of Tenofovir Alafenamide Mono-Fumarate: A Metastable Phase Featuring a Mixture of Co-Crystal and Salt. International Journal of Molecular Sciences. 2020; 21(23):9213. https://doi.org/10.3390/ijms21239213
Chicago/Turabian StyleWang, Jian-Wei, Lu Liu, Ka-Xi Yu, Hong-Zhen Bai, Jun Zhou, Wen-Hua Zhang, Xiurong Hu, and Guping Tang. 2020. "On the Single-Crystal Structure of Tenofovir Alafenamide Mono-Fumarate: A Metastable Phase Featuring a Mixture of Co-Crystal and Salt" International Journal of Molecular Sciences 21, no. 23: 9213. https://doi.org/10.3390/ijms21239213
APA StyleWang, J. -W., Liu, L., Yu, K. -X., Bai, H. -Z., Zhou, J., Zhang, W. -H., Hu, X., & Tang, G. (2020). On the Single-Crystal Structure of Tenofovir Alafenamide Mono-Fumarate: A Metastable Phase Featuring a Mixture of Co-Crystal and Salt. International Journal of Molecular Sciences, 21(23), 9213. https://doi.org/10.3390/ijms21239213