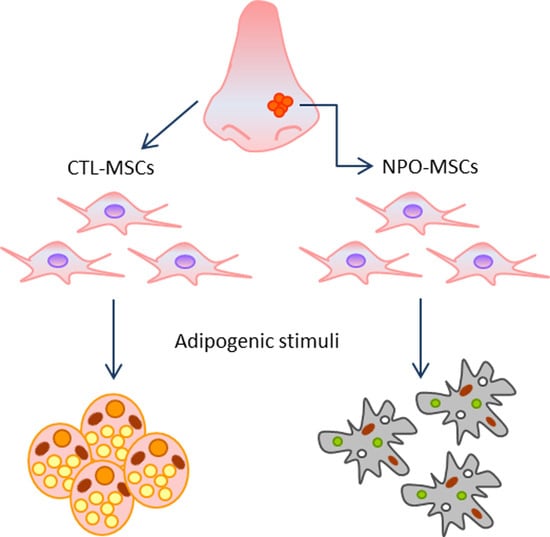
Deficit in Adipose Differentiation in Mesenchymal Stem Cells Derived from Chronic Rhinosinusitis Nasal Polyps Compared to Nasal Mucosal Tissue
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Mesenchymal Stem Cells (MSCs) Isolation and Culture
4.2. Flow Cytometry and Apoptosis
4.3. Annexin V/Propidium Iodide (PI) Staining Assay
4.4. Cell Proliferation Assay
4.5. Adipocyte Differentiation
4.6. Oil Red O Staining
4.7. RNA Isolation, Reverse Transcription and Quantitative RT-PCR
4.8. Statistics
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CRS | Chronic rhinosinusitis |
| CRSsNP | CRS without nasal polyposis |
| CRSwNP | CRS with nasal polyposis |
| MHC-II | class of major histocompatibility complex |
| FACS | Fluorescence-activated Cell Sorting |
| PPARg2 | peroxisome proliferator-activated receptors |
| FABP4 | fatty acid binding protein 4 |
| ADIPO-Q | adiponectin |
| ADSCs | Adipose-derived stem cells |
| MPC | Mesenchymal progenitor cells |
| ABC1 | ATP Binding Cassette Subfamily A Member 1 |
| FGF10 | Fibroblast Growth Factor 10 |
| KDR | Kinase Insert Domain Receptor |
| GDF6 | Growth Differentiation Factor 6 |
| HLA-DR | human leukocyte antigen-D related |
| PDL1-2 | Programmed death-ligand 1-2 |
| EDTA | Ethylenediaminetetraacetic acid |
| FITC | Fluorescein isothiocyanate |
| GADPH | Glyceraldehyde-3-phosphate dehydrogenase |
| MOPS | 3-(N-morpholino)propanesulfonic acid |
| PBS | Phosphate-buffered saline |
| MesenPRO RS™ | Mesenchymal Proliferation reduced serum Trade Mark |
References
- Schneider, S.; Campion, N.J.; Villazala-Merino, S.; Liu, D.T.; Bartosik, T.; Landegger, L.D.; Ahmadi, N.; Mueller, C.A.; Vyskocil, E.; Stanek, V.; et al. Associations between the Quality of Life and Nasal Polyp Size in Patients Suffering from Chronic Rhinosinusitis without Nasal Polyps, with Nasal Polyps or Aspirin-Exacerbated Respiratory Disease. J. Clin. Med. 2020, 9, 925. [Google Scholar] [CrossRef] [Green Version]
- Fokkens, W.J.; Lund, V.J.; Mullol, J.; Bachert, C.; Alobid, I.; Baroody, F.; Cohen, N.; Cervin, A.; Douglas, R.; Gevaert, P.; et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology 2012, 50, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Fokkens, W.J.; Lund, V.J.; Hopkins, C.; Hellings, P.W.; Kern, R.; Reitsma, S.; Toppila-Salmi, S.; Bernal-Sprekelsen, M.; Mullol, J.; Alobid, I.; et al. European position paper on rhinosinusitis and nasal polyps. Rhinology 2020, 58 (Suppl. S29), 1–464. [Google Scholar] [CrossRef]
- Koennecke, M.; Klimek, L.; Mullol, J.; Gevaert, P.; Wollenberg, B. Subtyping of polyposis nasi: Phenotypes, endotypes and comorbidities. Allergo J. Int. 2018, 27, 56–65. [Google Scholar] [CrossRef] [Green Version]
- Ahern, S.; Cervin, A. Inflammation and Endotyping in Chronic Rhinosinusitis-A Paradigm Shift. Medicina 2019, 55, 95. [Google Scholar] [CrossRef] [Green Version]
- Wynne, M.; Atkinson, C.; Schlosser, R.J.; Mulligan, J.K. Contribution of Epithelial Cell Dysfunction to the Pathogenesis of Chronic Rhinosinusitis with Nasal Polyps. Am. J. Rhinol. Allergy 2019, 33, 782–790. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, N.; Bo, M.; Holtappels, G.; Zheng, M.; Lou, H.; Wang, H.; Zhang, L.; Bachert, C. Diversity of TH cytokine profiles in patients with chronic rhinosinusitis: A multicenter study in Europe, Asia, and Oceania. J. Allergy Clin. Immunol. 2016, 138, 1344–1353. [Google Scholar] [CrossRef] [Green Version]
- Lou, H.; Zhang, N.; Bachert, C.; Zhang, L. Highlights of eosinophilic chronic rhinosinusitis with nasal polyps in definition, prognosis, and advancement. Int. Forum Allergy Rhinol. 2018, 8, 1218–1225. [Google Scholar] [CrossRef]
- Pelaia, C.; Calabrese, C.; Vatrella, A.; Busceti, M.T.; Garofalo, E.; Lombardo, N.; Terracciano, R.; Pelaia, G. Benralizumab: From the Basic Mechanism of Action to the Potential Use in the Biological Therapy of Severe Eosinophilic Asthma. Biomed. Res. Int. 2018, 2018, 4839230. [Google Scholar] [CrossRef]
- Ren, L.; Zhang, N.; Zhang, L.; Bachert, C. Biologics for the treatment of chronic rhinosinusitis with nasal polyps—State of the art. World Allergy Organ. J. 2019, 12, 100050. [Google Scholar] [CrossRef] [Green Version]
- Bachert, C.; Han, J.K.; Desrosiers, M.; Hellings, P.W.; Amin, N.; Lee, S.E.; Mullol, J.; Greos, L.S.; Bosso, J.V.; Laidlaw, T.M.; et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): Results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials. Lancet 2019, 394, 1638–1650. [Google Scholar] [CrossRef] [Green Version]
- Lombardo, N.; Pelaia, C.; Ciriolo, M.; Della Corte, M.; Piazzetta, G.; Lobello, N.; Viola, P.; Pelaia, G. Real-life effects of benralizumab on allergic chronic rhinosinusitis and nasal polyposis associated with severe asthma. Int. J. Immunopathol. Pharmacol. 2020, 34, 2058738420950851. [Google Scholar] [CrossRef]
- Gevaert, P.; Omachi, T.A.; Corren, J.; Mullol, J.; Han, J.; Lee, S.E.; Kaufman, D.; Ligueros-Saylan, M.; Howard, M.; Zhu, R.; et al. Efficacy and safety of omalizumab in nasal polyposis: 2 randomized phase 3 trials. J. Allergy Clin. Immunol. 2020, 146, 595–605. [Google Scholar] [CrossRef]
- Chiarella, E.; Lombardo, N.; Lobello, N.; Aloisio, A.; Aragona, T.; Pelaia, C.; Scicchitano, S.; Bond, H.M.; Mesuraca, M. Nasal Polyposis: Insights in Epithelial-Mesenchymal Transition and Differentiation of Polyp Mesenchymal Stem Cells. Int. J. Mol. Sci. 2020, 21, 6878. [Google Scholar] [CrossRef]
- Klimek, L.; Koennecke, M.; Mullol, J.; Hellings, P.W.; Wang, D.Y.; Fokkens, W.; Gevaert, P.; Wollenberg, B. A possible role of stem cells in nasal polyposis. Allergy 2017, 72, 1868–1873. [Google Scholar] [CrossRef] [Green Version]
- de Oliveira, P.W.; Pezato, R.; Agudelo, J.S.; Perez-Novo, C.A.; Berghe, W.V.; Câmara, N.O.; de Almeida, D.C.; Gregorio, L.C. Nasal Polyp-Derived Mesenchymal Stromal Cells Exhibit Lack of Immune-Associated Molecules and High Levels of Stem/Progenitor Cells Markers. Front. Immunol. 2017, 8, 39. [Google Scholar] [CrossRef]
- Cho, J.S.; Park, J.H.; Kang, J.H.; Kim, S.E.; Park, I.H.; Lee, H.M. Isolation and characterization of multipotent mesenchymal stem cells in nasal polyps. Exp. Biol. Med. 2015, 240, 185–193. [Google Scholar] [CrossRef] [Green Version]
- Koennecke, M.; Böscke, R.; Pfannerstill, A.C.; Reers, S.; Elsner, M.; Fell, B.; Richter, A.; Bruchhage, K.L.; Schumann, S.; Pries, R.; et al. Neuronal Differentiation Capability of Nasal Polyps of Chronic Rhinosinusitis. Arch. Immunol. Ther. Exp. 2017, 65, 431–443. [Google Scholar] [CrossRef]
- Di Vito, A.; Giudice, A.; Chiarella, E.; Malara, N.; Bennardo, F.; Fortunato, L. In Vitro Long-Term Expansion and High Osteogenic Potential of Periodontal Ligament Stem Cells: More Than a Mirage. Cell Transplant. 2019, 28, 129–139. [Google Scholar] [CrossRef] [Green Version]
- Chiarella, E.; Aloisio, A.; Scicchitano, S.; Lucchino, V.; Montalcini, Y.; Galasso, O.; Greco, M.; Gasparini, G.; Mesuraca, M.; Bond, H.M.; et al. ZNF521 Represses Osteoblastic Differentiation in Human Adipose-Derived Stem Cells. Int. J. Mol. Sci. 2018, 19, 4095. [Google Scholar] [CrossRef] [Green Version]
- Amable, P.R.; Teixeira, M.V.; Carias, R.B.; Granjeiro, J.M.; Borojevic, R. Gene expression and protein secretion during human mesenchymal cell differentiation into adipogenic cells. BMC Cell Biol. 2014, 15, 46. [Google Scholar] [CrossRef] [Green Version]
- Moseti, D.; Regassa, A.; Kim, W.-K. Molecular Regulation of Adipogenesis and Potential Anti-Adipogenic Bioactive Molecules. Int. J. Mol. Sci. 2016, 17, 124. [Google Scholar] [CrossRef] [Green Version]
- Prentice, K.J.; Saksi, J.; Hotamisligil, G.S. Adipokine FABP4 integrates energy stores and counterregulatory metabolic responses. J. Lipid Res. 2019, 60, 734–740. [Google Scholar] [CrossRef] [Green Version]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; et al. Minimal criteria for defining multipotent mesenchymal stromal cells: The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Afanasyev, B.V.; Elstner, E.E.; Zander, A.R.A.J. Friedenstein, founder of the mesenchymal stem cell concept. Cell. Ther. Transplant. 2009, 1, 3. [Google Scholar] [CrossRef]
- Berebichez-Fridman, R.; Montero-Olvera, P.R. Sources and Clinical Applications of Mesenchymal Stem Cells: State-of-the-art review. Sultan. Qaboos Univ. Med. J. 2018, 18, e264–e277. [Google Scholar] [CrossRef] [Green Version]
- Cristancho, A.; Lazar, M. Forming functional fat: A growing understanding of adipocyte differentiation. Nat. Rev. Mol. Cell Biol. 2011, 12, 722–734. [Google Scholar] [CrossRef]
- Bahmad, H.F.; Daouk, R.; Azar, J.; Sapudom, J.; Teo, J.C.M.; Abou-Kheir, W.; Al-Sayegh, M. Modeling Adipogenesis: Current and Future Perspective. Cells 2020, 9, 2326. [Google Scholar] [CrossRef]
- Chiarella, E.; Aloisio, A.; Codispoti, B.; Nappo, G.; Scicchitano, S.; Lucchino, V.; Montalcini, Y.; Camarotti, A.; Galasso, O.; Greco, M.; et al. ZNF521 Has an Inhibitory Effect on the Adipogenic Differentiation of Human Adipose-Derived Mesenchymal Stem Cells. Stem. Cell Rev. Rep. 2018, 14, 901–914. [Google Scholar] [CrossRef]
- Cho, K.S.; Park, H.Y.; Roh, H.J.; Bravo, D.T.; Hwang, P.H.; Nayak, J.V. Human ethmoid sinus mucosa: A promising novel tissue source of mesenchymal progenitor cells. Stem. Cell Res. Ther. 2014, 5, 15. [Google Scholar] [CrossRef] [Green Version]
- Bernaudo, F.; Monteleone, F.; Mesuraca, M.; Krishnan, S.; Chiarella, E.; Scicchitano, S.; Cuda, G.; Morrone, G.; Bond, H.M.; Gaspari, M. Validation of a novel shotgun proteomic workflow for the discovery of protein-protein interactions: Focus on ZNF521. J. Proteome Res. 2015, 14, 1888–1899. [Google Scholar] [CrossRef]
- Codispoti, B.; Rinaldo, N.; Chiarella, E.; Lupia, M.; Spoleti, C.B.; Marafioti, M.G.; Aloisio, A.; Scicchitano, S.; Giordano, M.; Nappo, G.; et al. Recombinant TAT-BMI-1 fusion protein induces ex vivo expansion of human umbilical cord blood-derived hematopoietic stem cells. Oncotarget 2017, 8, 43782–43798. [Google Scholar] [CrossRef] [Green Version]
- Chiarella, E.; Codispoti, B.; Aloisio, A.; Cosentino, E.G.; Scicchitano, S.; Montalcini, Y.; Lico, D.; Morrone, G.; Mesuraca, M.; Bond, H.M. Zoledronic acid inhibits the growth of leukemic MLL-AF9 transformed hematopoietic cells. Heliyon 2020, 6, e04020. [Google Scholar] [CrossRef]
- Di Vito, A.; Chiarella, E.; Baudi, F.; Scardamaglia, P.; Antonelli, A.; Giudice, D.; Barni, T.; Fortunato, L.; Giudice, A. Dose-dependent effects of zoledronic acid on human periodontal ligament stem cells: An in vitro pilot study. Cell Transplant. 2020, 29, 963689720948497. [Google Scholar] [CrossRef]
- Scicchitano, S.; Giordano, M.; Lucchino, V.; Montalcini, Y.; Chiarella, E.; Aloisio, A.; Codispoti, B.; Zoppoli, P.; Melocchi, V.; Bianchi, F.; et al. The stem cell-associated transcription co-factor, ZNF521, interacts with GLI1 and GLI2 and enhances the activity of the Sonic hedgehog pathway. Cell Death Dis. 2019, 10, 715. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiarella, E.; Lombardo, N.; Lobello, N.; Piazzetta, G.L.; Morrone, H.L.; Mesuraca, M.; Bond, H.M. Deficit in Adipose Differentiation in Mesenchymal Stem Cells Derived from Chronic Rhinosinusitis Nasal Polyps Compared to Nasal Mucosal Tissue. Int. J. Mol. Sci. 2020, 21, 9214. https://doi.org/10.3390/ijms21239214
Chiarella E, Lombardo N, Lobello N, Piazzetta GL, Morrone HL, Mesuraca M, Bond HM. Deficit in Adipose Differentiation in Mesenchymal Stem Cells Derived from Chronic Rhinosinusitis Nasal Polyps Compared to Nasal Mucosal Tissue. International Journal of Molecular Sciences. 2020; 21(23):9214. https://doi.org/10.3390/ijms21239214
Chicago/Turabian StyleChiarella, Emanuela, Nicola Lombardo, Nadia Lobello, Giovanna Lucia Piazzetta, Helen Linda Morrone, Maria Mesuraca, and Heather Mandy Bond. 2020. "Deficit in Adipose Differentiation in Mesenchymal Stem Cells Derived from Chronic Rhinosinusitis Nasal Polyps Compared to Nasal Mucosal Tissue" International Journal of Molecular Sciences 21, no. 23: 9214. https://doi.org/10.3390/ijms21239214
APA StyleChiarella, E., Lombardo, N., Lobello, N., Piazzetta, G. L., Morrone, H. L., Mesuraca, M., & Bond, H. M. (2020). Deficit in Adipose Differentiation in Mesenchymal Stem Cells Derived from Chronic Rhinosinusitis Nasal Polyps Compared to Nasal Mucosal Tissue. International Journal of Molecular Sciences, 21(23), 9214. https://doi.org/10.3390/ijms21239214