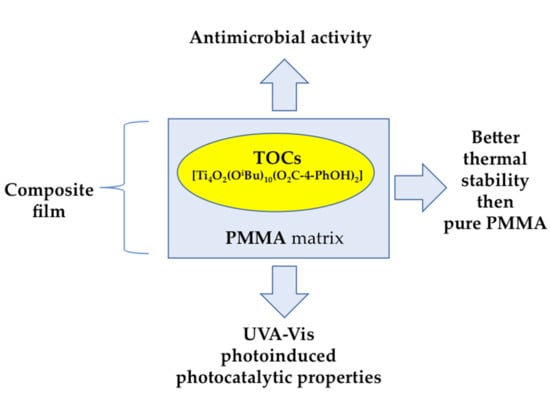
Oxo-Titanium(IV) Complex/Polymer Composites—Synthesis, Spectroscopic Characterization and Antimicrobial Activity Test
Abstract
:1. Introduction
2. Results
2.1. UV–Vis Diffuse Reflectance Spectra (UV-Vis-DRS) of the (1)–(4) Oxo-Complexes and HOMO-LUMO Gap Determination
2.2. (PMMA + TOC) Composites
2.3. Thermal Analysis of PMMA/TOC Composites
2.4. Estimation of Photocatalytic Activity of the (1)–(4) Oxo-Complexes
2.5. Antimicrobial Activity of (PMMA + TOCs) Composites
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Synthesis of Ti(IV) Oxo-Complexes (TOCs) and (PMMA +TOCs) Composites
4.3. Analytical Procedures
4.4. HOMO-LUMO Gap Determination
4.5. The Photocatalytic Activity Evaluation of (PMMA + TOCs) Composites
4.6. The Evaluation of Antimicrobial Properties of (PMMA + TOCs) Systems
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hashimoto, K.; Irie, H.; Fujishima, A. TiO2 Photocatalysis: A Historical Overview and Future Prospects. Jpn. J. Appl. Phys. 2005, 44, 8269–8285. [Google Scholar] [CrossRef]
- Chen, X.; Mao, S.S. Titanium Dioxide Nanomaterials: Synthesis, Properties, Modifications, and Applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef] [PubMed]
- Carp, O.; Huisman, C.L.; Reller, A. Photoinduced Reactivity of Titanium Dioxide. Prog. Solid State Chem. 2004, 32, 33–177. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X.X.; Tryk, D.A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–558. [Google Scholar] [CrossRef]
- Benedict, J.B.; Coppens, P. The Crystalline Nanocluster Phase as a Medium for Structural and Spectroscopic Studies of Light Absorption of Photosensitizer Dyes on Semiconductor Surfaces. J. Am. Chem. Soc. 2010, 132, 2938–2944. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Matthews, P.D.; Luo, H.-K.; Wright, D.S. Novel properties and potential applications of functional ligand-modified polyoxotitanate cages. Chem. Commun. 2016, 52, 11180–11190. [Google Scholar] [CrossRef] [Green Version]
- Assi, H.; Mouchaham, G.; Steunou, N.; Devic, T.; Serre, C. Titanium coordination compounds: From discrete metal complexes to metal–organic frameworks. Chem. Soc. Rev. 2017, 46, 3431–3452. [Google Scholar] [CrossRef]
- Benedict, J.B.; Freindorf, R.; Trzop, E.; Cogswell, J. Large Polyoxotitanate Clusters: Well-Defined Models for Pure-Phase TiO2Structures and Surfaces. J. Am. Chem. Soc. 2010, 132, 13669–13671. [Google Scholar] [CrossRef]
- Coppens, P.; Chen, Y.; Trzop, E. Crystallography and Properties of Polyoxotitanate Nanoclusters. Chem. Rev. 2014, 114, 9645–9661. [Google Scholar] [CrossRef]
- Rozes, L.; Sanchez, C. Titanium oxo-clusters: Precursors for a Lego-like construction of nanostructured hybrid materials. Chem. Soc. Rev. 2011, 40, 1006–1030. [Google Scholar] [CrossRef]
- Su, H.-C.; Wu, Y.-Y.; Hou, J.-L.; Zhang, G.-L.; Zhu, Q.-Y.; Dai, J. Dye molecule bonded titanium alkoxide: A possible new type of dye for sensitized solar cells. Chem. Commun. 2016, 52, 4072–4075. [Google Scholar] [CrossRef] [PubMed]
- Zou, D.-H.; Cui, L.-N.; Liu, P.-Y.; Yang, S.; Zhu, Q.-Y.; Dai, J. Triphenylamine derived titanium oxo clusters: An approach to effective organic–inorganic hybrid dyes for photoactive electrodes. Chem. Commun. 2018, 54, 9933–9936. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Zhang, Y.; Zou, H.-M.; Li, H.-M.; Cui, Y.; Jing, Q.-S.; Lu, H.-T. Modulating the band gap and photoelectrochemical activity of dicarboxylate-stabilized titanium-oxo clusters. Inorg. C. Acta 2018, 482, 16–22. [Google Scholar] [CrossRef]
- Macyk, W.; Szaciłowski, K.; Stochel, G.; Buchalska, M.; Kuncewicz, J.; Łabuz, P. Titanium(IV) complexes as direct TiO2 photosensitizers. Coord. Chem. Rev. 2010, 254, 2687–2701. [Google Scholar] [CrossRef]
- Hong, Z.-F.; Xu, S.-H.; Yan, Z.-H.; Lu, D.-F.; Kong, X.-J.; Long, L.-S.; Zheng, L.-S. A Large Titanium Oxo Cluster Featuring a Well-Defined Structural Unit of Rutile. Cryst. Growth Des. 2018, 18, 4864–4868. [Google Scholar] [CrossRef]
- Su, M.K.; Wu, Y.; Tan, W.; Wang, D.; Yuan, M.H. A monomeric bowl-like pyrogallol[4]arene Ti12 coordination complex. Chem. Commun. 2017, 53, 9598–9601. [Google Scholar] [CrossRef]
- Wu, Y.-Y.; Luo, W.; Wang, Y.-H.; Pu, Y.-Y.; Zhang, X.; You, L.-S.; Dai, J. Titanium–oxo–Clusters with Dicarboxylates: Single-Crystal Structure and Photochromic Effect. Inorg. Chem. 2012, 51, 8982–8988. [Google Scholar] [CrossRef]
- Luo, W.; Ge, G. Two Titanium-oxo-Clusters with Malonateand Succinate Ligands: Single-Crystal Structuresand Catalytic Property. J. Clust. Sci. 2016, 27, 635–643. [Google Scholar] [CrossRef]
- Kim, S.; Sarkar, D.; Kim, Y.; Park, M.H.; Yoon, M.; Kim, Y.; Kim, M. Synthesis of functionalized titanium-carboxylate molecular clusters and their catalytic activity. J. Ind. Eng. Chem. 2017, 53, 171–176. [Google Scholar] [CrossRef]
- Janek, M.; Muzioł, T.M.; Piszczek, P. Trinuclear Oxo-Titanium Clusters: Synthesis, Structure, and Photocatalytic Activity. Materials 2019, 12, 3195. [Google Scholar] [CrossRef] [Green Version]
- Janek, M.; Radtke, A.; Muzioł, T.M.; Jerzykiewicz, M.; Piszczek, P. Tetranuclear Oxo-Titanium Clusters with Different Carboxylate Aromatic Ligands: Optical Properties, DFT Calculations, and Photoactivity. Materials 2018, 11, 1661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janek, M.; Muzioł, T.; Piszczek, P. The structure and photocatalytic activity of the tetranuclear titanium(IV) oxo-complex with 4-aminobenzoate ligands. Polyhedron 2018, 141, 110–117. [Google Scholar] [CrossRef]
- Pathak, S.; Jana, B.; Mandal, M.; Mandal, V.; Ghorai, T.K. Antimicrobial activity study of a μ3-oxo bridged [Fe3O(PhCO2)6(MeOH)3](NO3)(MeOH)2] cluster. J. Mol. Struct. 2017, 1147, 480–486. [Google Scholar] [CrossRef]
- Gumerova1, N.I.; Al-Sayed, E.; Lukáš Krivosudský, L.; Čipčić-Paljetak, H.; Verbanac, D.; Rompel, A. Antibacterial Activity of Polyoxometalates Against Moraxella catarrhalis. Front. Chem. 2018, 6, 336. [Google Scholar] [CrossRef]
- Kumar, S.; Bhanjana, G.; Kumar, R.; Dilbaghi, N. Synthesis, Characterization and Antimicrobial Activity of Nano Titanium(IV) Mixed Ligand Complex. Mater. Focus 2013, 2, 475–481. [Google Scholar] [CrossRef]
- Kaushal, R.; Kumar, N.; Thakur, A.; Nehra, K.; Awasthi, P.; Kaushal, R.; Arora, S. Synthesis, Spectral Characterization, Antibacterial and Anticancer Activity of some Titanium Complexes. Anticancer Agents Med. Chem 2018, 18, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, R.; Thakur, S. Syntheses and Biological screening of Schiff base complexes of Titanium(IV). Chem. Eng. Trans. 2013, 32, 1801–1806. [Google Scholar] [CrossRef]
- Svensson, F.G.; Seisenbaeva, G.A.; Kessler, V.G. Mixed-Ligand Titanium “Oxo Clusters”: Structural Insights into the Formation and Binding of Organic Molecules and Transformation into Oxide Nanostructures on Hydrolysis and Thermolysis. Eur. J. Inorg. Chem. 2017, 35, 4117–4122. [Google Scholar] [CrossRef]
- Zafar, M.S. Prosthodontic Applications of PolymethylMethacrylate (PMMA): An Update. Polymers 2020, 12, 2299. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, X.; Zhang, J.; Zheng, Q.; Liu, B. Study on Antibacterial Property of PMMA Denture Base Materials with Negative Ion Powder. Mater. Sci. Eng. 2018, 301, 012034. [Google Scholar] [CrossRef]
- Chen, R.; Han, Z.; Huang, Z.; Karki, J.; Wang, C.; Zhu, B.; Zhang, X. Antibacterial activity, cytotoxicity and mechanical behaviour of nano-enhanced denture base resin with different kinds of inorganic antibacterial agents. Dent. Mol. J. 2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadagar, A.; Khalil, S.; Kassaee, M.Z.; Shahroudi, A.S.; Pourakbari, B.; Bahador, A. Antimicrobial properties of poly(methyl methacrylate) acrylic resins incorporated with silicon dioxide and titanium dioxide nanoparticles on cariogenic bacteria. J. Orthod. Sci. 2016, 5, 7–13. [Google Scholar] [CrossRef] [Green Version]
- De Mori, A.; Di Grgorio, E.; Kao, A.P.; Tozzi, G.; Barbu, E.; Sanghani-Kerai, A.; Draheim, R.R.; Rolado, M. Antibacterial PMMA Composite Cments with Tunable Thermal and Mechanical Properties. ACS Omega 2019, 4, 19664–19675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, M.; Arun, S.; Upadhyaya, P.; Pugazhenthi, G. Properties of PMMA/clay nanocomposites prepared using various compatibilizer. Int. J. Mech. Mat. Eng. 2015, 10, 7. [Google Scholar] [CrossRef]
- Gałka, P.; Kowalonek, J.; Kaczmarek, H. Thermogravimetric analysis of thermal stability of poly(methylmethacrylate) films modified with photoinitiators. J. Therm. Anal. Calorim. 2014, 115, 1387–1394. [Google Scholar] [CrossRef] [Green Version]
- Lu, P.; Huang, Q.; Mukherjee, A.; Hsieh, Y.-L. Effects of polymer matrices to the formation of silicon carbide (SiC) nanoporous fibers and nanowires under carbothermal reduction. J. Mater. Chem. 2011, 21, 1005–1012. [Google Scholar] [CrossRef]
- Elshereksi, N.W.; Mohamd, S.H.; Arifin, A.; Ishak, Z.A.M. Thermal Characterization of Poly(Methyl Methacrylate) Filled with Barium Titanate as Denture Base Material. J. Phys. Sci. 2014, 25, 15–27. [Google Scholar]
- ISO 10678:2010(E) Standards. Fine Ceramics (Advanced Ceramics, Advanced Technical Ceramics)—Determination of Photocatalytic Activity of Surfaces in an Aqueous Medium by Degradation of Methylene Blue. Available online: https://www.iso.org/standard/46019.html (accessed on 10 June 2020).
- Ehlert, M.; Radtke, A.; Topolski, A.; Śmigiel, J.; Piszczek, P. The Photocatalytic Activity of Titania Coatings Produced by Electrochemical and Chemical Oxidation of Ti6Al4V Substrate, Estimated According to ISO 10678:2010. Materials 2020, 13, 2649. [Google Scholar] [CrossRef]
- Patrick, W.; Dietmar, S. Photodegradation of rhodamine B In aqueous solution via SiO2@TiO2 nanosphares. J. Photochem. Photobiol. A Chem. 2007, 185, 19–25. [Google Scholar] [CrossRef]
- Liu, J.-X.; Gao, M.-Y.; Fang, W.-H.; Zhang, L.; Zhang, J. Bandgap Engineering of Titanium-Oxo Clusters: Labile Surface Sites Used for Ligand Substitution and Metal Incorporation. AngewandeChemie 2016, 128, 5246–5251. [Google Scholar] [CrossRef]
- Cui, Y.; Zou, G.-D.; Li, H.-M.; Huang, Y.; Fan, Y. 4-Chlorocalicylate-stabilized titanium-oxo clusters with structures mediated by tetrazole and their photophysical properties. Polyhedron 2019, 157, 177–182. [Google Scholar] [CrossRef]
- Habibi, S.; Jamshidi, M. Sol–gel synthesis of carbon-doped TiO2 nanoparticles based on microcrystalline cellulose for efficient photocatalytic degradation of methylene blue under visible light. J. Environ. Technol. 2020, 41, 3233–3247. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.-T.; Li, H.-M.; Zou, G.-D.; Cui, Y.; Huang, Y.; Fan, Y. Tioanium-oxo clusters functionalized with cateholate-tye ligands: Modulating the optical properties through charge-transfer transition. Dalton Trans. 2018, 47, 8158–8163. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Ghafoor, K.; Lee, J.; Feng, M.; Hong, J.; Lee, D.; Park, J. Bacterial inactivation in water, DNA strand breaking, and membrane damage induced by ultraviolet assisted titanium dioxide photocatalysis. Water Res. 2013, 47, 4403–4411. [Google Scholar] [CrossRef]
- Wu, M.J.; Bak, T.; O’Doherty, P.J.; Moffitt, M.C.; Nowotny, J.; Bailey, T.D.; Kersaitis, C. Photocatalysis of titanium dioxide for water disinfection: Challenges and future perspectives. Int. J. Photogramm. 2014, 973484. [Google Scholar] [CrossRef] [Green Version]
- Ramesh, T.; Nayak, B.; Amirbahman, A.; Tripp, C.P.; Mukhopadhyay, S. Application of ultraviolet light assisted titanium dioxide photocatalysis for food safety: A review. Innov. Food Sci. Emerg. Technol. 2016, 38, 105–115. [Google Scholar] [CrossRef] [Green Version]
- Maness, P.; Smolinski, S.; Blake, D.M.; Huang, Z.; Wolfrum, E.J.; Jacoby, W.A. Bactericidal activity of photocatalytic TiO2 reaction: Toward an understanding of its killing mechanism. Appl. Environ. Microbiol. 1999, 65, 4094–4098. [Google Scholar] [CrossRef] [Green Version]
- Su, W.; Wang, S.; Wang, X.; Fu, X.; Weng, J. Plasma pre-treatment and TiO2 coating of PMMA for the improvement of antibacterial properties. Surf. Coat. Technol. 2010, 205, 465–469. [Google Scholar] [CrossRef]
- Lin, H.; Xu, Z.; Wang, X.; Long, J.; Su, W.; Fu, X.; Lin, Q. Photocatalytic and antibacterial properties of medical-grade PVC material coated with TiO2 film. J. BiomedMater. Res. B Appl. Biomater. 2008, 87, 425–431. [Google Scholar] [CrossRef]
- Yu, J.C.; Ho, W.; Lin, J.; Yip, H.; Wong, P.K. Photocatalytic activity, antibacterial effect, and photoinduced hydrophilicity of TiO2 films coated on a stainless steel substrate. Environ. Sci. Technol. 2003, 37, 2296–2301. [Google Scholar] [CrossRef]
- Huang, Z.; Maness, P.C.; Blake, D.M.; Wolfrum, E.J.; Smolinski, S.L.; Jacoby, W.A. Bactericidalmode of titaniumdioxidephotocatalysis. J. Photochem. Photobiol. A Chem. 2000, 130, 163–170. [Google Scholar] [CrossRef]
- Pan, R.; Liu, Y.; Chen, W.; Dawson, G.; Wang, X.; Li, W.; Dong, B.; Zhu, Y. The toxicity evaluation of nano-trititanate with bactericidal properties in vitro. Nanotoxicology 2012, 6, 327–337. [Google Scholar] [CrossRef]
- Jesline, A.; John, N.P.; Narayanan, P.M.; Vani, C.; Murugan, S. Antimicrobial activity of zinc and titanium dioxide nanoparticles against biofilm-producing methicillin-resistant. Staphylococcus aureus. Appl. Nanosci. 2015, 5, 157–162. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, S.; Patil, S.; Ahire, M.; Kitture, R.; Kale, S.; Pardesi, K. Synthesis of silver nanoparticles using Dioscoreabulbifera tuberextract and evaluation of its synergistic potential in combination with antimicrobial agents. Int. J. Nanomed. 2012, 7, 483–496. [Google Scholar] [CrossRef] [Green Version]
- Inbaneson, S.J.; Ravikumar, S.; Manikandan, N. Antibacterial potential of silver nanoparticles against isolated urinary tract infectious bacterial pathogens. Appl. Nanosci. 2011, 1, 231–236. [Google Scholar] [CrossRef] [Green Version]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Ramirez, J.T. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, R.; Kumar, A.; Abraham, J. A biological approach to the synthesis of silver nanoparticles with Streptomyces sp. JAR1 and its antimicrobial activity. Sci. Pharm. 2013, 81, 607–621. [Google Scholar] [CrossRef] [Green Version]
- Jung, W.K.; Koo, H.C.; Kim, K.W.; Shin, S.; Kim, S.H.; Park, Y.H. Antibacterial activity and mechanism of action of the silver ion in Staphylococcus aureus and Escherichia coli. Appl. Environ. Microbiol. 2008, 74, 2171–2178. [Google Scholar] [CrossRef] [Green Version]
- Lok, C.N.; Ho, C.M.; Chen, R.; He, Q.Y.; Yu, W.Y.; Sun, H. Proteomic analysis of the mode of antibacterial action of silver nanoparticles. J. Proteome Res. 2006, 5, 916–924. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, H.S.; Ryu, D.S.; Choi, S.J.; Lee, D.S. Antibacterial activity of silver-nanoparticles against Staphylococcus aureus and Escherichia coli. Korean J. Microbiol. Biotechnol. 2011, 39, 77–85. [Google Scholar]
- Li, J.; Rong, K.; Zhao, H.; Li, F.; Lu, Z.; Chen, R. Highly selective antibacterial activities of silver nanoparticles against Bacillus subtilis. J. Nanosci. Nanotechnol. 2013, 13, 6806–6813. [Google Scholar] [CrossRef] [PubMed]
- Bajpaia, V.K.; Kamleb, M.; Shuklaa, S.; Mahatoc, D.K.; Chandrad, P.; Hwange, S.K.; Kumarb, P.; Huhe, Y.S.; Han, Y.-K. Prospects of using nanotechnology for food preservation, safety, and security. J. Food Drug Anal. 2018, 26, 1201–1214. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Ouyang, S.; Liu, L.; Reunchan, P.; Umezawa, N.; Ye, J. Recent advances in TiO2-based photocatalysis. J. Mat. Chem. A. 2014, 2, 12642–12661. [Google Scholar] [CrossRef]
- ISO 22196:2011 Standards. Measurement of Antibacterial Activity on Plastics and Other Non-Porous Surfaces. Available online: https://www.kieferusa.com/wp-content/uploads/2019/07/MRSA-ISO-22196.pdf (accessed on 3 June 2019).
- Isola, G.; Alibrandi, A.; Currò, M.; Matarese, M.; Ricc, A.S.; Matarese, G.; Ientile, R.; Kocher, T. Evaluation of salivary and serum ADMA levels in patients with periodontal and cardiovascular disease as subclinical marker of cardiovascular risk. J. Periodontol. 2020, 91, 1076–1084. [Google Scholar] [CrossRef]
- Isola, G.; Polizzi, A.; Patini, R.; Ferlito, S.; Alibrandi, A.; Palazzo, G. Association among serum and salivary A. actinomycetemcomitans specific immunoglobulin antibodies and periodontitis. BMC Oral Health 2020, 20, 283. [Google Scholar] [CrossRef]







| 4:1 Molar Ratio | Isolated | ||
|---|---|---|---|
| Ti(OR)4 | HOOCR’ | Solid Product | Solvent |
| Ti(OiPr)4 | HOOC-4-PhNH2 | (1) | THF/iPrOH |
| Ti(OiPr)4 | HOOC-4-PhOH | (2) | THF/iPrOH |
| Ti(OiBu)4 | HOOC-4-PhNH2 | (3) | Toluene |
| Ti(OiBu)4 | HOOC-4-PhOH | (4) | THF/iBuOH |
| (1) | (2) | (3) | (4) | |||||
|---|---|---|---|---|---|---|---|---|
| Modes | IR | R | IR | R | IR | R | IR | R |
| ν(OH) ν(NH2) | 3200–3450 | 3162 (w) | 3300–3500 | 3200–3450 | 3175 (w) | 3300–3500 | ||
| ν(CC) (Ph) | 1622 (m) 1601 (m) | 1603 (s) | 1622 (m) 1603 (m) | 1595 (s) | 1621 (m) 1603 (m) | 1602 (s) | 1622 (m) 1603 (m) | 1594 (s) |
| δ(NH2) | 1587 (m) | 1589 (m) | ||||||
| νas(COO) | 1533 (m) 1516 (m) | 1524 (m) | 1536 (m) 1516 (m) | 1519 (w) | 1523 (m) | 1524 (m) | 1536 (m) 1510 (w) | 1518 (w) |
| νs(COO) | 1462 (w) | 1436 (m) | 1464 (w) | 1450 (w) | 1497 (m) | 1459 (w) | 1498 (m) | 1445 (w) |
| δ(NH2) | 1362 (m) | 1306 (m) | 1299 (m) | 1300 (m) | ||||
| ν(C-O) + δ(CC) | 1014 (s) | 1016 (m) | 1036 (m) | 1016 (m) | ||||
| ν(Ti-OR) + ν(CC) + δ(CH) | 986 (m) 949 (m) | 856 (m) | 988 (m) 951 (m) | 838 (m) | 971 (m) 948 (w) | 856 (m) | 988 (m) 951 (w) | 838 (m) |
| ν(CH) 1,4-Ph | 780 (m) | 782 (m) | 784 (s) | 782 (m) | ||||
| ν(Ti3-(μ3-O) | 750 (w) 728 (w) | 747 (vw) 719 (w) | 755 (vw) 727 (vw) | 748 (vw) | ||||
| ν(Ti2-(μ-O) | 703 (m) | 690 (vw) | 702 (m) | 687 (vw) | ||||
| ν(Ti4-(μ4-O) | 639 (m) | 623 (w) | 634 (m) | 610 (vw) | ||||
| δ(CCC) | 683 (w) | 622 (m) | 701 (w) | 678 (m) | ||||
| 666 (m) | 605 (w) | 638 (m) | 638 (w) | |||||
| 622 (m) | 629 (w) | |||||||
| ν(Ti3-(μ3-O) | 561 (w) | 564 (w) | 539 (vw) | |||||
| ν(Ti4-(μ4-O) | 532 (m) | 548 (vw) | 556 (m) | 536 (vw) | ||||
| 532 (w) | 510 (w) | |||||||
| ν(Ti3-(μ3-O) | 486 (m) | 483 (m) | ||||||
| 417 (w) | 419 (w) | |||||||
| ν(Ti3-(μ3-O) | 342 (vw) | 350 (vw) | ||||||
| TOCs | m/z | Fragmentation Ion | Intensity (%) |
|---|---|---|---|
| [Ti3O(OiPr)8(O2C-4-PhNH2)2] (1) | 768 | (Ti3O(OiPr)8(O2C-4-PhNH2))+ | 8 |
| 550 | (Ti3O(OiPr)2(O2C-4-PhNH2))+ | 36 | |
| [Ti3O(OiPr)8(O2C-4-PhOH)2] (2) | 847 | (Ti3O(OiPr)7(O2C-4-PhOH)2)+ | 26 |
| 769 | (Ti3O(OiPr)8(O2C-4-PhOH))+ | 90 | |
| [Ti4O2(OiBu)10(O2C-4-PhNH2)2] (3) | 1080 | (Ti4O2(OiBu)8(O2C-4-PhNH2)2)+ | 5 |
| 881 | (Ti4O2(OiBu)9)+ | 5 | |
| [Ti4O2(OiBu)10(O2C-4-PhOH)2] (4) | 1229 | (Ti4O2(OiBu)10(O2CPhOH)2) + H+ | 8 |
| 945 | (Ti4O2(OiBu)8(O2CPhOH))+ | 10 |
| DSC | TGA | |||||
|---|---|---|---|---|---|---|
| Composite | Tg/°C | T/°C | Tm/°C | Stage I Tmax/°C/Δm/% | Stage II Tmax/°C/Δm/% | Solid Residue at 450 °C (%) |
| PMMA | 99.6 | 150.8 | 368.6 | 194.9/12 | 365.1/85 | 3 |
| (PMMA+(1)) | 100.6 | - | 370.4 | 188.2/12 | 367.8/70 | 18 |
| (PMMA+(2)) | 101.2 | - | 375.7 | 183.9/11 | 365.4/74 | 15 |
| (PMMA+(3)) | 99.0 | - | 377.8 | 159.9/15 | 372.7/76 | 9 |
| (PMMA+(4)) | 115.2 | - | 364.5 | 171.0/10 | 374.0/79 | 11 |
| Composite | MB Decolorization a (%) | ΔA180 | ΔA180 in Reference to PMMA |
| PMMA+(1) | 6.70 | 0.067 | –0.001 |
| PMMA+(2) | 5.50 | 0.055 | –0.013 |
| PMMA+(3) | 9.97 | 0.100 | 0.032 |
| PMMA+(4) | 10.42 | 0.104 | 0.036 |
| PMMA(irradiated) | 6.76 | 0.068 | - |
| Composite | RhB Decolorization b (%) | ΔA180 | ΔA180 in Reference to PMMA |
| PMMA+(1) | 0.73 | 0.007 | –0.004 |
| PMMA+(2) | 0.92 | 0.009 | –0.002 |
| PMMA+(3) | 1.14 | 0.011 | 0 |
| PMMA+(4) | 1.75 | 0.018 | 0.007 |
| PMMA(irradiated) | 1.14 | 0.011 | - |
| Microorganisms | PMMA+ (1) | PMMA+ (2) | PMMA+ (3) | PMMA+(4) | ||||
|---|---|---|---|---|---|---|---|---|
| R | R% | R | R% | R | R% | R | R% | |
| E. coli ATCC 8739 | 2.5 | 99.6 | 6.0 | >99.99 | 6.0 | >99.99 | 6.0 | >99.99 |
| E. coli ATCC 25922 | 3.3 | 99.95 | 6.0 | >99.99 | 6.0 | >99.99 | 6.0 | >99.99 |
| S. aureus ATCC 6538 | 6.0 | >99.99 | 6.0 | >99.99 | 6.0 | >99.99 | 6.0 | >99.99 |
| S. aureus ATCC 25923 | 6.0 | >99.99 | 6.0 | >99.99 | 6.0 | >99.99 | 6.0 | >99.99 |
| C. albicans ATCC 10231 | 1.7 | 85.0 | 6.0 | >99.99 | 6.0 | >99.99 | 6.0 | >99.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piszczek, P.; Kubiak, B.; Golińska, P.; Radtke, A. Oxo-Titanium(IV) Complex/Polymer Composites—Synthesis, Spectroscopic Characterization and Antimicrobial Activity Test. Int. J. Mol. Sci. 2020, 21, 9663. https://doi.org/10.3390/ijms21249663
Piszczek P, Kubiak B, Golińska P, Radtke A. Oxo-Titanium(IV) Complex/Polymer Composites—Synthesis, Spectroscopic Characterization and Antimicrobial Activity Test. International Journal of Molecular Sciences. 2020; 21(24):9663. https://doi.org/10.3390/ijms21249663
Chicago/Turabian StylePiszczek, Piotr, Barbara Kubiak, Patrycja Golińska, and Aleksandra Radtke. 2020. "Oxo-Titanium(IV) Complex/Polymer Composites—Synthesis, Spectroscopic Characterization and Antimicrobial Activity Test" International Journal of Molecular Sciences 21, no. 24: 9663. https://doi.org/10.3390/ijms21249663
APA StylePiszczek, P., Kubiak, B., Golińska, P., & Radtke, A. (2020). Oxo-Titanium(IV) Complex/Polymer Composites—Synthesis, Spectroscopic Characterization and Antimicrobial Activity Test. International Journal of Molecular Sciences, 21(24), 9663. https://doi.org/10.3390/ijms21249663