Adipose-Derived Mesenchymal Stem Cell Chondrospheroids Cultured in Hypoxia and a 3D Porous Chitosan/Chitin Nanocrystal Scaffold as a Platform for Cartilage Tissue Engineering
Abstract
:1. Introduction
2. Results
2.1. Influence of Hypoxia on hASC Chondrospheroid Generation
2.2. Chondrogenic Differentiation of hASC Spheroids in Hypoxia
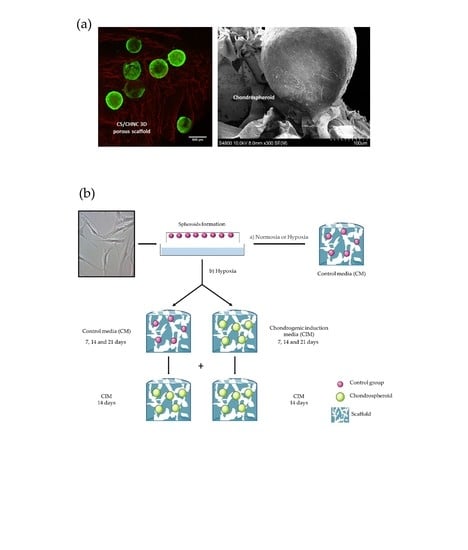
2.3. hASC Spheroid Adhesion, Viability and Chondrogenic Differentiation in the 3D Porous CS/CHNC Scaffold under Hypoxia
3. Discussion
4. Materials and Methods
4.1. hASC Spheroids Formation and Chondrogenic Differentiation
4.2. Chondrospheroid Volume Quantification
4.3. Chondrospheroid Viability
4.4. Histological Analysis
4.5. Western Blot
4.6. Synthesis of the 3D Porous Genipin-Crosslinked CS/CHNC Scaffold
4.7. hASC Spheroids Adhesion and Viability in the 3D Porous CS/CHNC Scaffold
4.8. Chondrogenic Differentiation in the 3D Porous CS/CHNC Scaffolds
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ishiguro, N.; Kojima, T.; Robin Poole, A. Mechanism of cartilage destruction in osteoarthritis. Nagoya J. Med. Sci. 2002, 65, 73–84. [Google Scholar] [PubMed]
- Tan, A.R.; Hung, C.T. Concise Review: Mesenchymal StemCells for Functional Cartilage Tissue Engineering: Taking Cues from Chondrocyte-Based Constructs. Stem Cells Transl. Med. 2016, 2, 614–624. [Google Scholar] [CrossRef] [PubMed]
- Camarero-Espinosa, S.; Rothen-Rutishauser, B.; Foster, E.J.; Weder, C. Articular cartilage: From formation to tissue engineering. Biomater. Sci. 2016, 4, 734–767. [Google Scholar] [CrossRef] [PubMed]
- Tran-Khanh, N.; Hoemann, C.D.; McKee, M.D.; Henderson, J.E.; Buschmann, M.D. Aged bovine chondrocytes display a diminished capacity to produce a collagen-rich, mechanically functional cartilage extracellular matrix. J. Orthop. Res. 2005, 23, 1354–1362. [Google Scholar] [CrossRef] [PubMed]
- Islam, A.; Hansen, A.K.; Mennan, C.; Zubiaurre-Martinez, I. Mesenchymal stromal cells from human umbilical cord stromal cells display poor chondrogenic potential in scaffold-free three dimentional cultures: A comparative study. Eur. Cells Mater. 2016, 31, 407–424. [Google Scholar] [CrossRef] [PubMed]
- Kokai, L.E.; Marra, K.; Rubin, J.P. Adipose stem cells: Biology and clinical applications for tissue repair and regeneration. Transl. Res. 2014, 163, 399–408. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, M.; Guo, Q.-Y.; Lu, S.-B.; Peng, J. Mesenchymal Stem Cells for Treating Articular Cartilage Defects and Osteoarthritis. Cell Transplant. 2015, 24, 1661–1678. [Google Scholar] [CrossRef] [Green Version]
- Izadpanah, R.; Trygg, C.; Patel, B.; Kriedt, C.; Dufour, J.; Gimble, J.M.; Bunnell, B.A. Biologic properties of mesenchymal stem cells derived from bone marrow and adipose tissue. J. Cell Biochem. 2006, 99, 1285–1297. [Google Scholar] [CrossRef] [Green Version]
- Strioga, M.; Viswanathan, S.; Darinskas, A.; Slaby, O.; Michalek, J. Same or Not the Same? Comparison of Adipose Tissue-Derived Versus Bone Marrow-Derived Mesenchymal Stem and Stromal Cells. Stem Cells Dev. 2012, 21, 2724–2752. [Google Scholar] [CrossRef]
- Li, C.; Wu, X.; Tong, J.; Yang, X.; Zhao, J.; Zheng, Q.; Zhao, G.; Ma, Z. Comparative analysis of human mesenchymal stem cells from bone marrow and adipose tissue under xeno-free conditions for cell therapy. Stem Cell Res. Ther. 2015, 6, 55. [Google Scholar] [CrossRef] [Green Version]
- Frese, L.; Dijkman, P.E.; Hoerstrup, S.P. Adipose tissue-derived stem cells in regenerative medicine. Transfus. Med. Hemotherapy 2016, 43, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Hao, T.; Chen, J.; Zhi, S.; Zhang, Q.; Chen, G.; Yu, F. Comparison of bone marrow-vs. Adipose tissue-derived mesenchymal stem cells for attenuating liver fibrosis. Exp. Ther. Med. 2017, 14, 5956–5964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schon, B.S.; Schrobback, K.; Van Der Ven, M.; Stroebel, S.; Hooper, G.J.; Woodfield, T.B.F. Validation of a high-throughput microtissue fabrication process for 3D assembly of tissue engineered cartilage constructs. Cell Tissue Res. 2012, 347, 629–642. [Google Scholar] [CrossRef] [PubMed]
- Santo, V.E.; Popa, E.G.; Mano, J.F.; Gomes, M.E.; Reis, R.L. Natural assembly of platelet lysate-loaded nanocarriers into enriched 3D hydrogels for cartilage regeneration. Acta Biomater. 2015, 19, 56–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Y.; Ma, J.; Zhang, X.; Li, H.; Jiang, L.; Qin, J. Hypoxia combined with spheroid culture improves cartilage specific function in chondrocytes. Integr. Biol. 2015, 7, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Wan Safwani, W.K.Z.; Choi, J.R.; Yong, K.W.; Ting, I.; Mat Adenan, N.A.; Pingguan-Murphy, B. Hypoxia enhances the viability, growth and chondrogenic potential of cryopreserved human adipose-derived stem cells. Cryobiology 2017, 75, 91–99. [Google Scholar] [CrossRef]
- Choi, J.R.; Yong, K.W.; Wan Safwani, W.K.Z. Effect of hypoxia on human adipose-derived mesenchymal stem cells and its potential clinical applications. Cell. Mol. Life Sci. 2017, 74, 2587–2600. [Google Scholar] [CrossRef]
- Jurgens, W.J.F.M.; Lu, Z.; Zandieh-Doulabi, B.; Kuik, D.J.; Ritt, M.J.P.F.; Helder, M.N. Hyperosmolarity and hypoxia induce chondrogenesis of adipose-derived stem cells in a collagen type 2 hydrogel. J. Tissue Eng. Regen. Med. 2012, 6, 570–578. [Google Scholar] [CrossRef]
- Tibbitt, M.W.; Anseth, K.S. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol. Bioeng. 2009, 103, 655–663. [Google Scholar] [CrossRef] [Green Version]
- Zou, F.; Li, R.; Jiang, J.; Mo, X.; Gu, G.; Guo, Z.; Chen, Z. Mechanical enhancement and in vitro biocompatibility of nanofibrous collagen-chitosan scaffolds for tissue engineering. J. Biomater. Sci. Polym. Ed. 2017, 28, 2255–2270. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, S.; Chen, D. Preparation and characterization of chitosan based injectable hydrogels enhanced by chitin. J. Mech. Behav. Biomed. Mater. 2017, 65, 466–477. [Google Scholar] [CrossRef] [PubMed]
- Zubillaga, V.; Salaberria, A.M.; Palomares, T.; Kootala, S.; Labidi, J.; Fernandes, S.C.M. Chitin nanoforms provide mechanical and topological cues to support growth of human adipose stem cells in chitosan matrices. Biomacromolecules 2018, 19, 3000–3012. [Google Scholar] [CrossRef] [PubMed]
- Salaberria, A.M.; Diaz, R.H.; Labidi, J.; Fernandes, S.C.M. Preparing valuable renewable nanocomposite films based exclusively on oceanic biomass – Chitin nanofillers and chitosan. React. Funct. Polym. 2015, 89, 31–39. [Google Scholar] [CrossRef]
- Salaberria, A.M.; Labidi, J.; Fernandes, S.C.M. Different routes to turn chitin into stunning nano-objects. Eur. Polym. J. 2015, 68, 503–515. [Google Scholar] [CrossRef]
- Salaberria, A.M.; Diaz, R.H.; Labidi, J.; Fernandes, S.C.M. Role of chitin nanocrystals and nanofibers on physical, mechanical and functional properties in thermoplastic starch films. Food Hydrocoll. 2015, 46, 93–102. [Google Scholar] [CrossRef]
- Salaberria, A.M.; Fernandes, S.C.M.; Diaz, R.H.; Labidi, J. Processing of α-chitin nanofibers by dynamic high pressure homogenization: Characterization and antifungal activity against A. niger. Carbohydr. Polym. 2015, 116, 286–291. [Google Scholar] [CrossRef]
- Pangon, A.; Saesoo, S.; Saengkrit, N.; Ruktanonchai, U.; Intasanta, V. Hydroxyapatite-hybridized chitosan/chitin whisker bionanocomposite fibers for bone tissue engineering applications. Carbohydr. Polym. 2016, 144, 419–427. [Google Scholar] [CrossRef]
- Wu, H.; Liu, J.; Fang, Q.; Xiao, B.; Wan, Y. Establishment of nerve growth factor gradients on aligned chitosan-polylactide /alginate fibers for neural tissue engineering applications. Colloids Surfaces B Biointerfaces 2017, 160, 598–609. [Google Scholar] [CrossRef]
- Varoni, E.M.; Vijayakumar, S.; Canciani, E.; Cochis, A.; De Nardo, L.; Lodi, G.; Rimondini, L.; Cerruti, M. Chitosan-Based Trilayer Scaffold for Multitissue Periodontal Regeneration. J. Dent. Res. 2018, 97, 303–311. [Google Scholar] [CrossRef]
- Mujtaba, M.; Salaberria, A.M.; Andres, M.A.; Kaya, M.; Gunyakti, A.; Labidi, J. Utilization of flax (Linum usitatissimum) cellulose nanocrystals as reinforcing material for chitosan films. Int. J. Biol. Macromol. 2017, 104, 944–952. [Google Scholar] [CrossRef]
- Tang, F.; Lv, L.; Lu, F.; Rong, B.; Li, Z.; Lu, B.; Yu, K.; Liu, J.; Dai, F.; Wu, D.; et al. Preparation and characterization of N-chitosan as a wound healing accelerator. Int. J. Biol. Macromol. 2016, 93, 1295–1303. [Google Scholar] [CrossRef] [PubMed]
- Dhivya, S.; Keshav Narayan, A.; Logith Kumar, R.; Viji Chandran, S.; Vairamani, M.; Selvamurugan, N. Proliferation and differentiation of mesenchymal stem cells on scaffolds containing chitosan, calcium polyphosphate and pigeonite for bone tissue engineering. Cell Prolif. 2018, 51, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez, I.; Elvenes, J.; Olsen, R.; Bertheussen, K.; Johansen, O. Redifferentiation of in vitro expanded adult articular chondrocytes by combining the hanging-drop cultivation method with hypoxic environment. Cell Transplant. 2008, 17, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Buravkova, L.B.; Andreeva, E.R.; Gogvadze, V.; Zhivotovsky, B. Mesenchymal stem cells and hypoxia: Where are we? Mitochondrion 2014, 19, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Marga, F.; Neagu, A.; Kosztin, I.; Forgacs, G. Developmental biology and tissue engineering. Birth Defects Res. C Embryo Today 2007, 81, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Ruedel, A.; Hofmeister, S.; Bosserhoff, A.K. Development of a model system to analyze chondrogenic differentiation of mesenchymal stem cells. Int. J. Clin. Exp. Pathol. 2013, 6, 3042–3048. [Google Scholar]
- Gionet-Gonzales, M.A.; Leach, J.K. Engineering principles for guiding spheroid function in the regeneration of bone, cartilage, and skin. Biomed. Mater. 2018, 13, 034109. [Google Scholar] [CrossRef]
- Yoon, H.H.; Bhang, S.H.; Shin, J.Y.; Shin, J.; Kim, B.-S. Enhanced Cartilage Formation via Three-Dimensional Cell Engineering of Human Adipose-Derived Stem Cells. Tissue Eng. Part A 2012, 18, 1949–1956. [Google Scholar] [CrossRef] [Green Version]
- Stubbs, S.L.; Hsiao, S.T.F.; Peshavariya, H.M.; Lim, S.Y.; Dusting, G.J.; Dilley, R.J. Hypoxic Preconditioning Enhances Survival of Human Adipose-Derived Stem Cells and Conditions Endothelial Cells In Vitro. Stem Cells Dev. 2012, 21, 1887–1896. [Google Scholar] [CrossRef]
- Kakudo, N.; Morimoto, N.; Ogawa, T.; Taketani, S.; Kusumoto, K. Hypoxia enhances proliferation of human adipose-derived stem cells via HIF-1α activation. PLoS ONE 2015, 10, e0139890. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.R.; Pingguan-Murphy, B.; Wan Abas, W.A.B.; Noor Azmi, M.A.; Omar, S.Z.; Chua, K.H.; Wan Safwani, W.K.Z. Hypoxia Promotes Growth and Viability of Human Adipose- Derived Stem Cells with Increased Growth Factors. J. Asian Sci. Res. 2014, 448, 218–224. [Google Scholar]
- Hoyer, M.; Meier, C.; Breier, A.; Hahner, J.; Heinrich, G.; Drechsel, N.; Meyer, M.; Rentsch, C.; Garbe, L.A.; Ertel, W.; et al. In vitro characterization of self-assembled anterior cruciate ligament cell spheroids for ligament tissue engineering. Histochem. Cell Biol. 2014, 143, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Hartanto, Y.; Zhang, H. Advances in multicellular spheroids formation. J. R. Soc. Interface 2017, 14, 20160877. [Google Scholar] [CrossRef] [PubMed]
- Markway, B.D.; Tan, G.K.; Brooke, G.; Hudson, J.E.; Cooper-White, J.J.; Doran, M.R. Enhanced Chondrogenic Differentiation of Human Bone Marrow-Derived Mesenchymal Stem Cells in Low Oxygen Environment Micropellet Cultures. Cell Transplant. 2010, 19, 29–42. [Google Scholar] [CrossRef]
- Goude, M.C.; McDevitt, T.C.; Temenoff, J.S. Chondroitin sulfate microparticles modulate transforming growth factor-β1-induced chondrogenesis of human mesenchymal stem cell spheroids. Cells Tissues Organs 2014, 199, 117–130. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Tao, Y.; Wang, J.; Liang, C.; Wang, J.; Li, H.; Chen, Q. Roles of FGF-2 and TGF-beta/FGF-2 on differentiation of human mesenchymal stem cells towards nucleus pulposus-like phenotype. Growth Factors 2015, 33, 23–30. [Google Scholar] [CrossRef]
- Hering, T.M.; Wirthlin, L.; Ravindran, S.; McAlinden, A. Changes in type II procollagen isoform expression during chondrogenesis by disruption of an alternative 5′ splice site within Col2a1 exon 2. Matrix Biol. 2014, 36, 51–63. [Google Scholar] [CrossRef]
- McAlinden, A. Alternative splicing of type II procollagen: IIB or not IIB? Connect. Tissue Res. 2014, 55, 165–176. [Google Scholar] [CrossRef] [Green Version]
- Galeano-Garces, C.; Camilleri, E.T.; Riester, S.M.; Dudakovic, A.; Larson, D.R.; Qu, W.; Smith, J.; Dietz, A.B.; Im, H.J.; Krych, A.J.; et al. Molecular Validation of Chondrogenic Differentiation and Hypoxia Responsiveness of Platelet-Lysate Expanded Adipose Tissue–Derived Human Mesenchymal Stromal Cells. Cartilage 2017, 8, 283–299. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Li, X.; Jing, X.; Li, M.; Ren, Y.; Chen, J.; Yang, C.; Wu, H.; Guo, F. Hypoxia promotes maintenance of the chondrogenic phenotype in rat growth plate chondrocytes through the HIF-1α/YAP signaling pathway. Int. J. Mol. Med. 2018, 42, 3181–3192. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Yoon, S.M.; Song, S.U.; Park, S.G.; Kim, W.S.; Park, I.G.; Lee, J.; Sung, J.H. Hypoxia suppresses spontaneous mineralization and osteogenic differentiation of mesenchymal stem cells via IGFBP3 Up-regulation. Int. J. Mol. Sci. 2016, 17, 1389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.H.; Chang, C.C.; Shieh, M.J.; Wang, J.P.; Chen, Y.T.; Young, T.H.; Hung, S.C. Hypoxia enhances Chondrogenesis and prevents terminal differentiation through Pi3k/Akt/FoxO dependent anti-Apoptotic effect. Sci. Rep. 2013, 3, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Malladi, P.; Chiou, M.; Bekerman, E.; Giaccia, A.J.; Longaker, M.T. In Vitro Expansion of Adipose-Derived Adult Stromal Cells in Hypoxia Enhances Early Chondrogenesis. Tissue Eng. 2007, 13, 2981–2993. [Google Scholar] [CrossRef] [PubMed]
- Noh, H.K.; Lee, S.W.; Kim, J.M.; Oh, J.E.; Kim, K.H.; Chung, C.P.; Choi, S.C.; Park, W.H.; Min, B.M. Electrospinning of chitin nanofibers: Degradation behavior and cellular response to normal human keratinocytes and fibroblasts. Biomaterials 2006, 27, 3934–3944. [Google Scholar] [CrossRef] [PubMed]
- Dash, M.; Chiellini, F.; Ottenbrite, R.M.; Chiellini, E. Chitosan—A versatile semi-synthetic polymer in biomedical applications. Prog. Polym. Sci. 2011, 36, 981–1014. [Google Scholar] [CrossRef]
- Zhang, K.; Yan, S.; Li, G.; Cui, L.; Yin, J. In-situ birth of MSCs multicellular spheroids in poly(L-glutamic acid)/chitosan scaffold for hyaline-like cartilage regeneration. Biomaterials 2015, 71, 24–34. [Google Scholar] [CrossRef]
- Huang, G.S.; Dai, L.G.; Yen, B.L.; Hsu, S. hui Spheroid formation of mesenchymal stem cells on chitosan and chitosan-hyaluronan membranes. Biomaterials 2011, 32, 6929–6945. [Google Scholar] [CrossRef]
- Murphy KC, F.S. and L.K. Human mesenchymal stem cell spheroids in fibrin hydrogels exhibit improved cell survival and potential for bone healing. Cell Tissue Res. 2014, 357, 91–99. [Google Scholar] [CrossRef]
- Karlsson, J.O.; Ostwald, K.; Kåbjörn, C.; Andersson, M. A method for protein assay in laemmli buffer. Anal. Biochem. 1994, 219, 144–146. [Google Scholar] [CrossRef]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zubillaga, V.; Alonso-Varona, A.; Fernandes, S.C.M.; Salaberria, A.M.; Palomares, T. Adipose-Derived Mesenchymal Stem Cell Chondrospheroids Cultured in Hypoxia and a 3D Porous Chitosan/Chitin Nanocrystal Scaffold as a Platform for Cartilage Tissue Engineering. Int. J. Mol. Sci. 2020, 21, 1004. https://doi.org/10.3390/ijms21031004
Zubillaga V, Alonso-Varona A, Fernandes SCM, Salaberria AM, Palomares T. Adipose-Derived Mesenchymal Stem Cell Chondrospheroids Cultured in Hypoxia and a 3D Porous Chitosan/Chitin Nanocrystal Scaffold as a Platform for Cartilage Tissue Engineering. International Journal of Molecular Sciences. 2020; 21(3):1004. https://doi.org/10.3390/ijms21031004
Chicago/Turabian StyleZubillaga, Veronica, Ana Alonso-Varona, Susana C. M. Fernandes, Asier M. Salaberria, and Teodoro Palomares. 2020. "Adipose-Derived Mesenchymal Stem Cell Chondrospheroids Cultured in Hypoxia and a 3D Porous Chitosan/Chitin Nanocrystal Scaffold as a Platform for Cartilage Tissue Engineering" International Journal of Molecular Sciences 21, no. 3: 1004. https://doi.org/10.3390/ijms21031004
APA StyleZubillaga, V., Alonso-Varona, A., Fernandes, S. C. M., Salaberria, A. M., & Palomares, T. (2020). Adipose-Derived Mesenchymal Stem Cell Chondrospheroids Cultured in Hypoxia and a 3D Porous Chitosan/Chitin Nanocrystal Scaffold as a Platform for Cartilage Tissue Engineering. International Journal of Molecular Sciences, 21(3), 1004. https://doi.org/10.3390/ijms21031004