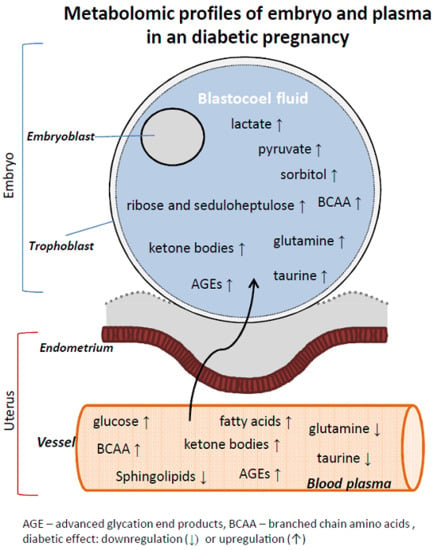
Metabolic Profiling in Blastocoel Fluid and Blood Plasma of Diabetic Rabbits
Abstract
:1. Introduction
2. Results
2.1. Carbohydrates and Energy Metabolites in Maternal Plasma and Blastocysts Cavity Fluid Samples from Diabetic Rabbits
2.2. Lipid Metabolites in Maternal Plasma and Blastocysts Cavity Fluid Samples from Diabetic Rabbits
2.3. Amino Acids and Their Derivatives in Maternal Plasma and Blastocyst Cavity Fluid Samples from Diabetic Rabbits
2.4. Purine- and Pyrimidine-related Biochemicals in Maternal Plasma and Blastocyst Cavity Fluid Samples from Diabetic Rabbits
2.5. Vitamins and Cofactors in Maternal Plasma and Blastocyst Cavity Fluid Samples from Diabetic Rabbits
3. Discussion
3.1. Maternal Diabetes Mellitus Increases Glycolysis and Pentose Metabolism in Preimplantation Blastocysts
3.2. Diabetic Dysregulation of Maternal and Embryonic Lipid Metabolism
3.3. Amino Acid Metabolism is Differently Regulated in the Maternal Tissues and Embryo
4. Materials and Methods
4.1. Alloxan Treatment
4.2. Collection of Plasma Samples and Blastocoel Fluids for Metabolomics Analysis
4.3. Metabolic Analysis
4.4. Statistics
4.5. Data Availability
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 1,5-AG | 1,5 anhydroglucitol |
| ADP | adenosine 5’-diphosphate |
| AGE | advanced glycation end products |
| BCAA | branched chain amino acid |
| BF | blastocysts cavity fluid, blastocoel fluid |
| BHBA | body3-hydroxybutyrate |
| CML | carboxymethyllysine |
| CPT1 | carnitine palmitoyltransferase 1 |
| d6 | day 6 |
| dpc | day post-coitum |
| DT1 | diabetes mellitus type 1 |
| ESI | electrospray ionization |
| FAD | flavin adenine dinucleotide |
| GDM | gestational diabetes mellitus |
| GLUT | Glucose transporter protein |
| IVF | in vitro fertilisation |
| NADPH | nicotinamide adenine dinucleotide phosphate |
| p38 MAPK | p38 mitogen-activated protein kinase |
| p.c. | post coitum |
| PPP | pentose phosphate pathway |
| TCA | tricarboxylic acid cycle |
References
- Barnett, D.K.; Bavister, B.D. What is the relationship between the metabolism of preimplantation embryos and their developmental competence? Mol. Reprod. Dev. 1996, 43, 105–133. [Google Scholar] [CrossRef]
- Hu, K.; Yu, Y. Metabolite availability as a window to view the early embryo microenvironment in vivo. Mol. Reprod. Dev. 2017, 84, 1027–1038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leese, H.J.; Conaghan, J.; Martin, K.L.; Hardy, K. Early human embryo metabolism. BioEssays 1993, 15, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Leese, H.J. Metabolism of the preimplantation embryo: 40 years on. Reproduction (Camb. Engl.) 2012, 143, 417–427. [Google Scholar] [CrossRef] [Green Version]
- Uyar, A.; Seli, E. Metabolomic assessment of embryo viability. Semin. Reprod. Med. 2014, 32, 141–152. [Google Scholar] [CrossRef] [Green Version]
- Nicholson, J.K.; Lindon, J.C.; Holmes, E. ‘Metabonomics’: Understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data. Xenobiotica Fate Foreign Compd. Biol. Syst. 1999, 29, 1181–1189. [Google Scholar] [CrossRef]
- Allen, J.; Davey, H.M.; Broadhurst, D.; Heald, J.K.; Rowland, J.J.; Oliver, S.G. High-throughput classification of yeast mutants for functional genomics using metabolic footprinting. Nat. Biotechnol. 2003, 21, 692–696. [Google Scholar] [CrossRef]
- Lindon, J.C.; Holmes, E.; Nicholson, J.K. So what’s the deal with metabonomics? Anal. Chem. 2003, 75, 384A–391A. [Google Scholar] [CrossRef]
- Bracewell-Milnes, T.; Saso, S.; Abdalla, H.; Nikolau, D.; Norman-Taylor, J.; Johnson, M. Metabolomics as a tool to identify biomarkers to predict and improve outcomes in reproductive medicine: A systematic review. Hum. Reprod. Update 2017, 23, 723–736. [Google Scholar] [CrossRef] [Green Version]
- Dunn, W.B.; Broadhurst, D.I.; Atherton, H.J.; Goodacre, R.; Griffin, J.L. Systems level studies of mammalian metabolomes: The roles of mass spectrometry and nuclear magnetic resonance spectroscopy. Chem. Soc. Rev. 2011, 40, 387–426. [Google Scholar] [CrossRef]
- Roberts, L.D.; Souza, A.L.; Gerszten, R.E.; Clish, C.B. Targeted metabolomics. Curr. Protoc. Mol. Biol. 2012, 98, 30.2.1–30.2.24. [Google Scholar] [CrossRef] [PubMed]
- McCabe, C.F.; Perng, W. Metabolomics of Diabetes in Pregnancy. Curr. Diab. Rep. 2017, 17, 57. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Scholl, T.O.; Leskiw, M.; Savaille, J.; Stein, T.P. Differences in maternal circulating fatty acid composition and dietary fat intake in women with gestational diabetes mellitus or mild gestational hyperglycemia. Diabetes Care 2010, 33, 2049–2054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dani, C.; Bresci, C.; Berti, E.; Ottanelli, S.; Mello, G.; Mecacci, F. Metabolomic profile of term infants of gestational diabetic mothers. The journal of maternal-fetal & neonatal medicine. J. Matern. Fetal. Neonatal. Med. 2014, 27, 537–542. [Google Scholar] [CrossRef]
- Pinto, J.; Almeida, L.M.; Martins, A.S.; Duarte, D.; Barros, A.S.; Galhano, E. Prediction of Gestational Diabetes through NMR Metabolomics of Maternal Blood. J. Proteome Res. 2015, 14, 2696–2706. [Google Scholar] [CrossRef]
- Baskind, N.E.; McRae, C.; Sharma, V.; Fisher, J. Understanding subfertility at a molecular level in the female through the application of nuclear magnetic resonance (NMR) spectroscopy. Hum. Reprod. Update 2011, 17, 228–241. [Google Scholar] [CrossRef] [Green Version]
- Jungheim, E.S.; Moley, K.H. The impact of type 1 and type 2 diabetes mellitus on the oocyte and the preimplantation embryo. Semin. Reprod. Med. 2008, 26, 186–195. [Google Scholar] [CrossRef]
- Ramin, N.; Thieme, R.; Fischer, S.; Schindler, M.; Schmidt, T.; Fischer, B. Maternal diabetes impairs gastrulation and insulin and IGF-I receptor expression in rabbit blastocysts. Endocrinology 2010, 151, 4158–4167. [Google Scholar] [CrossRef] [Green Version]
- Pantaleon, M.; Kaye, P.L. Glucose transporters in preimplantation development. Rev. Reprod. 1998, 3, 77–81. [Google Scholar] [CrossRef]
- Purcell, S.H.; Moley, K.H. Glucose transporters in gametes and preimplantation embryos. Trends Endocrinol. Metab. 2009, 20, 483–489. [Google Scholar] [CrossRef]
- Moley, K.H.; Chi, M.M.; Mueckler, M.M. Maternal hyperglycemia alters glucose transport and utilization in mouse preimplantation embryos. Am. J. Physiol. 1998, 275, E38–E47. [Google Scholar] [CrossRef] [PubMed]
- Knott, R.M.; Robertson, M.; Muckersie, E.; Forrester, J.V. Regulation of glucose transporters (GLUT-1 and GLUT-3) in human retinal endothelial cells. Biochem. J. 1996, 318 Pt 1, 313–317. [Google Scholar] [CrossRef] [Green Version]
- Sasson, S.; Ashhab, Y.; Melloul, D.; Cerasi, E. Autoregulation of glucose transport: Effects of glucose on glucose transporter expression and cellular location in muscle. Adv. Exp. Med. Biol. 1993, 334, 113–127. [Google Scholar] [CrossRef]
- Conaghan, J.; Handyside, A.H.; Winston, R.M.; Leese, H.J. Effects of pyruvate and glucose on the development of human preimplantation embryos in vitro. J. Reprod. Fertil. 1993, 99, 87–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butcher, L.; Coates, A.; Martin, K.L.; Rutherford, A.J.; Leese, H.J. Metabolism of pyruvate by the early human embryo. Biol. Reprod. 1998, 58, 1054–1056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dumollard, R.; Ward, Z.; Carroll, J.; Duchen, M.R. Regulation of redox metabolism in the mouse oocyte and embryo. Development (Camb. Engl.) 2007, 134, 455–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, P.; Reece, E.A.; Wang, F.; Gabbay-Benziv, R. Decoding the oxidative stress hypothesis in diabetic embryopathy through proapoptotic kinase signaling. Am. J. Obs. Gynecol. 2015, 212, 569–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gauster, M.; Majali-Martinez, A.; Maninger, S.; Gutschi, E.; Greimel, P.H.; Ivanisevic, M. Maternal Type 1 diabetes activates stress response in early placenta. Placenta 2017, 50, 110–116. [Google Scholar] [CrossRef]
- Kim, W.J.; Park, C.Y. 1,5-Anhydroglucitol in diabetes mellitus. Endocrine 2013, 43, 33–40. [Google Scholar] [CrossRef]
- Ying, L.; Ma, X.; Yin, J.; Wang, Y.; He, X.; Peng, J. The metabolism and transport of 1,5-anhydroglucitol in cells. Acta Diabetol 2018, 55, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.S.M.; Ho, E.C.M.; Lam, K.S.L.; Chung, S.K. Contribution of polyol pathway to diabetes-induced oxidative stress. J. Am. Soc. Nephrol. 2003, 14, S233–S236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gugliucci, A. Formation of Fructose-Mediated Advanced Glycation End Products and Their Roles in Metabolic and Inflammatory Diseases. Adv. Nutr. (BethesdaMd.) 2017, 8, 54–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haucke, E.; Navarrete Santos, A.; Simm, A.; Henning, C.; Glomb, M.A.; Gürke, J. Accumulation of advanced glycation end products in the rabbit blastocyst under maternal diabetes. Reproduction (Camb. Engl.) 2014, 148, 169–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, W.; Wang, R.-S.; Handy, D.E.; Loscalzo, J. NAD(H) and NADP(H) Redox Couples and Cellular Energy Metabolism. Antioxid. Redox Signal. 2018, 28, 251–272. [Google Scholar] [CrossRef] [PubMed]
- Stanton, R.C. Glucose-6-phosphate dehydrogenase, NADPH, and cell survival. Iubmb Life 2012, 64, 362–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, P.; Du, W.; Wu, M. Regulation of the pentose phosphate pathway in cancer. Protein Cell 2014, 5, 592–602. [Google Scholar] [CrossRef] [Green Version]
- Javed, M.H.; Wright, R.W. Determination of pentose phosphate and Embden-Meyerhof pathway activities in bovine embryos. Theriogenology 1991, 35, 1029–1037. [Google Scholar] [CrossRef]
- Schindler, M.; Pendzialek, M.; Navarrete Santos, A.; Plösch, T.; Seyring, A.; Gürke, J.; Haucke, E.; Knelangen, J.M.; Fischer, B.; Santos, A.N. Maternal diabetes leads to unphysiological high lipid accumulation in rabbit preimplantation embryos. Endocrinology 2014, 155, 1498–1509. [Google Scholar] [CrossRef] [Green Version]
- Jungheim, E.S.; Macones, G.A.; Odem, R.R.; Patterson, B.W.; Moley, K.H. Elevated serum α-linolenic acid levels are associated with decreased chance of pregnancy after in vitro fertilization. Fertil. Steril. 2011, 96, 880–883. [Google Scholar] [CrossRef] [Green Version]
- Mai, M.; Tönjes, A.; Kovacs, P.; Stumvoll, M.; Fiedler, G.M.; Leichtle, A.B. Serum levels of acylcarnitines are altered in prediabetic conditions. PLoS ONE 2013, 8, e82459. [Google Scholar] [CrossRef] [Green Version]
- Indiveri, C.; Iacobazzi, V.; Tonazzi, A.; Giangregorio, N.; Infantino, V.; Convertini, P. The mitochondrial carnitine/acylcarnitine carrier: Function, structure and physiopathology. Mol. Asp. Med. 2011, 32, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Koves, T.R.; Li, P.; An, J.; Akimoto, T.; Slentz, D.; Ilkayeva, O. Peroxisome proliferator-activated receptor-gamma co-activator 1alpha-mediated metabolic remodeling of skeletal myocytes mimics exercise training and reverses lipid-induced mitochondrial inefficiency. J. Biol. Chem. 2005, 280, 33588–33598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schindler, M.; Pendzialek, M.; Grybel, K.J.; Seeling, T.; Gürke, J.; Fischer, B. Adiponectin stimulates lipid metabolism via AMPK in rabbit blastocysts. Hum. Reprod. (Oxf. Engl.) 2017, 32, 1382–1392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, S.K.; Kannan, K.; Lim, G. Ketosis (acetoacetate) can generate oxygen radicals and cause increased lipid peroxidation and growth inhibition in human endothelial cells. Free Radic. Biol. Med. 1998, 25, 1083–1088. [Google Scholar] [CrossRef]
- White, R.L.; Wittenberg, B.A. Mitochondrial NAD(P)H, ADP, oxidative phosphorylation, and contraction in isolated heart cells. Am. J. Physiol. Heart Circ. Physi. 2000, 279, H1849–H1857. [Google Scholar] [CrossRef] [Green Version]
- Moley, K.H.; Vaughn, W.K.; Diamond, M.P. Manifestations of diabetes mellitus on mouse preimplantation development: Effect of elevated concentration of metabolic intermediates. Hum. Reprod. (Oxf. Engl.) 1994, 9, 113–121. [Google Scholar] [CrossRef]
- Khanmohammadi, N.; Movahedin, M.; Safari, M.; Sameni, H.R.; Yousefi, B.; Jafari, B. Effect of L-carnitine on in vitro developmental rate, the zona pellucida and hatching of blastocysts and their cell numbers in mouse embryos. Int. J. Reprod. Biomed. (YazdIran) 2016, 14, 649–656. [Google Scholar] [CrossRef] [Green Version]
- Kyvelidou, C.; Sotiriou, D.; Antonopoulou, T.; Tsagkaraki, M.; Tserevelakis, G.J.; Filippidis, G. l-Carnitine affects preimplantation embryo development toward infertility in mice. Reproduction (Camb. Engl.) 2016, 152, 283–291. [Google Scholar] [CrossRef] [Green Version]
- Lowe, J.L.; Bartolac, L.K.; Bathgate, R.; Grupen, C.G. Supplementation of culture medium with L-carnitine improves the development and cryotolerance of in vitro-produced porcine embryos. Reprod. Fertil. Dev. 2017, 29, 2357–2366. [Google Scholar] [CrossRef]
- Knitlova, D.; Hulinska, P.; Jeseta, M.; Hanzalova, K.; Kempisty, B.; Machatkova, M. Supplementation of l-carnitine during in vitro maturation improves embryo development from less competent bovine oocytes. Theriogenology 2017, 102, 16–22. [Google Scholar] [CrossRef]
- Koeberle, A.; Pergola, C.; Shindou, H.; Koeberle, S.C.; Shimizu, T.; Laufer, S.A. Role of p38 mitogen-activated protein kinase in linking stearoyl-CoA desaturase-1 activity with endoplasmic reticulum homeostasis. FASEB J. 2015, 29, 2439–2449. [Google Scholar] [CrossRef] [PubMed]
- Melland-Smith, M.; Ermini, L.; Chauvin, S.; Craig-Barnes, H.; Tagliaferro, A.; Todros, T. Disruption of sphingolipid metabolism augments ceramide-induced autophagy in preeclampsia. Autophagy 2015, 11, 653–669. [Google Scholar] [CrossRef] [PubMed]
- Yazama, H.; Kitatani, K.; Fujiwara, K.; Kato, M.; Hashimoto-Nishimura, M.; Kawamoto, K. Dietary glucosylceramides suppress tumor growth in a mouse xenograft model of head and neck squamous cell carcinoma by the inhibition of angiogenesis through an increase in ceramide. J. Clin. Oncol. 2015, 20, 438–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weissgerber, T.L.; Mudd, L.M. Preeclampsia and diabetes. Curr. Diab. Rep. 2015, 15, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vestgaard, M.; Sommer, M.C.; Ringholm, L.; Damm, P.; Mathiesen, E.R. Prediction of preeclampsia in type 1 diabetes in early pregnancy by clinical predictors: A systematic review. J. Matern. Fetal. Neonatal. Med. 2018, 31, 1933–1939. [Google Scholar] [CrossRef] [PubMed]
- Albaghdadi, A.J.H.; Kan, F.W.K. Endometrial receptivity defects and impaired implantation in diabetic NOD mice. Biol. Reprod. 2012, 87, 30. [Google Scholar] [CrossRef] [Green Version]
- Giesbertz, P.; Daniel, H. Branched-chain amino acids as biomarkers in diabetes. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 48–54. [Google Scholar] [CrossRef]
- Bloomgarden, Z. Diabetes and branched-chain amino acids: What is the link? J. Diabetes 2018, 10, 350–352. [Google Scholar] [CrossRef] [Green Version]
- Gürke, J.; Hirche, F.; Thieme, R.; Haucke, E.; Schindler, M.; Stangl, G.I. Maternal Diabetes Leads to Adaptation in Embryonic Amino Acid Metabolism during Early Pregnancy. PLoS ONE 2015, 10, e0127465. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Han, Q.; Liu, Y.; Sun, C.; Gang, X.; Wang, G. The Relationship between Branched-Chain Amino Acid Related Metabolomic Signature and Insulin Resistance: A Systematic Review. J. Diabetes Res. 2016, 2016, 2794591. [Google Scholar] [CrossRef]
- Holeček, M. Branched-chain amino acids in health and disease: Metabolism, alterations in blood plasma, and as supplements. Nutr. Metab. 2018, 15, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edwards, L.J.; Williams, D.A.; Gardner, D.K. Intracellular pH of the mouse preimplantation embryo: Amino acids act as buffers of intracellular pH. Hum. Reprod. (Oxf. Engl.) 1998, 13, 3441–3448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Winkle, L.J. Amino acid transport regulation and early embryo development. Biol. Reprod. 2001, 64, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Richards, T.; Wang, F.; Liu, L.; Baltz, J.M. Rescue of postcompaction-stage mouse embryo development from hypertonicity by amino acid transporter substrates that may function as organic osmolytes. Biol. Reprod. 2010, 82, 769–777. [Google Scholar] [CrossRef]
- Lane, M.; Gardner, D.K. Nonessential amino acids and glutamine decrease the time of the first three cleavage divisions and increase compaction of mouse zygotes in vitro. J. Assist. Reprod. Genet. 1997, 14, 398–403. [Google Scholar] [CrossRef] [Green Version]
- Martin, P.M.; Sutherland, A.E.; van Winkle, L.J. Amino acid transport regulates blastocyst implantation. Biol. Reprod. 2003, 69, 1101–1108. [Google Scholar] [CrossRef] [Green Version]
- Chatot, C.L.; Lawry, J.R.; Germain, B.; Ziomek, C.A. Analysis of glutaminase activity and RNA expression in preimplantation mouse embryos. Mol. Reprod. Dev. 1997, 47, 248–254. [Google Scholar] [CrossRef]
- Petters, R.M.; Johnson, B.H.; Reed, M.L.; Archibong, A.E. Glucose, glutamine and inorganic phosphate in early development of the pig embryo in vitro. J. Reprod. Fertil. Fertil. 1990, 89, 269–275. [Google Scholar] [CrossRef] [Green Version]
- McKiernan, S.H.; Bavister, B.D.; Tasca, R.J. Energy substrate requirements for in-vitro development of hamster 1- and 2-cell embryos to the blastocyst stage. Hum. Reprod. (Oxf. Engl.) 1991, 6, 64–75. [Google Scholar] [CrossRef]
- Miyoshi, K.; Abeydeera, L.R.; Okuda, K.; Niwa, K. Effects of osmolarity and amino acids in a chemically defined medium on development of rat one-cell embryos. J. Reprod. Fertil. Fertil. 1995, 103, 27–32. [Google Scholar] [CrossRef] [Green Version]
- Hansen, J.M.; Harris, C. Glutathione during embryonic development. Biochim. Biophys. Acta 2015, 1850, 1527–1542. [Google Scholar] [CrossRef] [PubMed]
- Dumoulin, J.C.; van Wissen, L.C.; Menheere, P.P.; Michiels, A.H.; Geraedts, J.P.; Evers, J.L. Taurine acts as an osmolyte in human and mouse oocytes and embryos. Biol. Reprod. 1997, 56, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Dumoulin, J.C.; Evers, J.L.; Bakker, J.A.; Bras, M.; Pieters, M.H.; Geraedts, J.P. Temporal effects of taurine on mouse preimplantation development in vitro. Hum. Reprod. (Oxf. Engl.) 1992, 7, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Barnett, D.K.; Bavister, B.D. Hypotaurine requirement for in vitro development of golden hamster one-cell embryos into morulae and blastocysts, and production of term offspring from in vitro-fertilized ova. Biol. Reprod. 1992, 47, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Casslén, B.G. Free amino acids in human uterine fluid. Possible role of high taurine concentration. J. Reprod. Med. 1987, 32, 181–184. [Google Scholar] [PubMed]
- Harris, S.E.; Gopichandran, N.; Picton, H.M.; Leese, H.J.; Orsi, N.M. Nutrient concentrations in murine follicular fluid and the female reproductive tract. Theriogenology 2005, 64, 992–1006. [Google Scholar] [CrossRef]
- Leese, H.J.; Hugentobler, S.A.; Gray, S.M.; Morris, D.G.; Sturmey, R.G.; Whitear, S.L. Female reproductive tract fluids: Composition, mechanism of formation and potential role in the developmental origins of health and disease. Reprod. Fertil. Dev. 2008, 20, 1–8. [Google Scholar] [CrossRef]
- Kermack, A.J.; Finn-Sell, S.; Cheong, Y.C.; Brook, N.; Eckert, J.J.; Macklon, N.S. Amino acid composition of human uterine fluid: Association with age, lifestyle and gynaecological pathology. Hum. Reprod. (Oxf. Engl.) 2015, 30, 917–924. [Google Scholar] [CrossRef] [Green Version]
- Baltz, J.M. Osmoregulation and cell volume regulation in the preimplantation embryo. In Current Topics in Developmental Biology; Elsevier: Amsterdam, The Netherlands, 2001; pp. 55–106. [Google Scholar]
- Steeves, C.L.; Hammer, M.-A.; Walker, G.B.; Rae, D.; Stewart, N.A.; Baltz, J.M. The glycine neurotransmitter transporter GLYT1 is an organic osmolyte transporter regulating cell volume in cleavage-stage embryos. Proc. Natl. Acad. Sci. USA 2003, 100, 13982–13987. [Google Scholar] [CrossRef] [Green Version]
- Redel, B.K.; Spate, L.D.; Lee, K.; Mao, J.; Whitworth, K.M.; Prather, R.S. Glycine supplementation in vitro enhances porcine preimplantation embryo cell number and decreases apoptosis but does not lead to live births. Mol. Reprod. Dev. 2016, 83, 246–258. [Google Scholar] [CrossRef]
- Herrick, J.R.; Lyons, S.M.; Greene, A.F.; Broeckling, C.D.; Schoolcraft, W.B.; Krisher, R.L. Direct and Osmolarity-Dependent Effects of Glycine on Preimplantation Bovine Embryos. PLoS ONE 2016, 11, e0159581. [Google Scholar] [CrossRef]
- Li, S.; Guo, Q.; Wang, Y.-M.; Li, Z.-Y.; Kang, J.-D.; Yin, X.-J. Glycine treatment enhances developmental potential of porcine oocytes and early embryos by inhibiting apoptosis. Anim. Sci. J. 2018, 96, 2427–2437. [Google Scholar] [CrossRef] [PubMed]
- Locasale, J.W. Serine, glycine and one-carbon units: Cancer metabolism in full circle. Nat. Rev. Cancer 2013, 13, 572–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tedeschi, P.M.; Markert, E.K.; Gounder, M.; Lin, H.; Dvorzhinski, D.; Dolfi, S.C. Contribution of serine, folate and glycine metabolism to the ATP, NADPH and purine requirements of cancer cells. Cell Death Dis. 2013, 4, e877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salbaum, J.M.; Kappen, C. Responses of the embryonic epigenome to maternal diabetes. Birth Defects Res. Part A Clin. Mol. Teratol. 2012, 94, 770–781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosen, E.D.; Kaestner, K.H.; Natarajan, R.; Patti, M.-E.; Sallari, R.; Sander, M. Epigenetics and Epigenomics: Implications for Diabetes and Obesity. Diabetes 2018, 67, 1923–1931. [Google Scholar] [CrossRef] [Green Version]
- Stuart, T.J.; O’Neill, K.; Condon, D.; Sasson, I.; Sen, P.; Xia, Y. Diet-induced obesity alters the maternal metabolome and early placenta transcriptome and decreases placenta vascularity in the mouse. Biol. Reprod. 2018, 98, 795–809. [Google Scholar] [CrossRef]
- Rousseau-Ralliard, D.; Couturier-Tarrade, A.; Thieme, R.; Brat, R.; Rolland, A.; Boileau, P. A short periconceptional exposure to maternal type-1 diabetes is sufficient to disrupt the feto-placental phenotype in a rabbit model. Mol. Cell. Endocrinol. 2019, 480, 42–53. [Google Scholar] [CrossRef]
- Starikov, R.; Inman, K.; Chen, K.; Lopes, V.; Coviello, E.; Pinar, H. Comparison of placental findings in type 1 and type 2 diabetic pregnancies. Placenta 2014, 35, 1001–1006. [Google Scholar] [CrossRef]
- Spange, S.; Wagner, T.; Heinzel, T.; Krämer, O.H. Acetylation of non-histone proteins modulates cellular signalling at multiple levels. Int. J. Biochem. Cell Biol. 2009, 41, 185–198. [Google Scholar] [CrossRef]
- Choudhary, C.; Kumar, C.; Gnad, F.; Nielsen, M.L.; Rehman, M.; Walther, T.C. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science 2009, 325, 834–840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schindler, M.; Fischer, S.; Thieme, R.; Fischer, B.; Navarrete Santos, A. cAMP-responsive element binding protein: A vital link in embryonic hormonal adaptation. Endocrinology 2013, 154, 2208–2221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischer, B.; Chavatte-Palmer, P.; Viebahn, C.; Navarrete Santos, A.; Duranthon, V. Rabbit as a reproductive model for human health. Reproduction (Camb. Engl.) 2012, 144, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Number of Biochemicals | |||||
|---|---|---|---|---|---|
| Total | Not Altered (p > 0.05) | Altered (p ≤ 0.05) | Diabetic Effect | ||
| Increased | Decreased | ||||
| Blastocoel fluid | 284 | 149 | 135 | 117 | 18 |
| Plasma | 597 | 258 | 339 | 232 | 107 |
| Sub Pathway | Biochemical Name | Plasma | BF |
|---|---|---|---|
| Glycolysis, Gluconeogenesis and Pyruvate Metabolism | 1,5-anhydroglucitol (1,5-AG) | 0.16 | 0.29 |
| glucose | 3.35 | 1.38 | |
| pyruvate | 2.24 | 1.65 | |
| lactate | 1.40 | 1.60 | |
| Pentose Metabolism | ribose | n.d. | 1.67 |
| xylose | 1.24 | 1.47 | |
| arabinose | 1.79 | 1.82 | |
| arabitol/xylitol | 1.93 | 2.13 | |
| arabonate/xylonate | 1.72 | 1.60 | |
| sedoheptulose | 1.14 | 2.27 | |
| Fructose, Mannose and Galactose Metabolism | fructose | 8.34 | n.d. |
| mannitol/sorbitol | 2.66 | 1.68 | |
| mannose | 1.83 | n.d. | |
| Advanced Glycation End-product | N6-carboxymethyllysine | 2.65 | n.d. |
| TCA Cycle | citrate | 1.20 | 1.69 |
| aconitate [cis or trans] | 1.82 | 1.89 | |
| isocitrate | n.d. | 2.01 | |
| alpha-ketoglutarate | 0.92 | 1.25 | |
| succinylcarnitine (C4-DC) | 1.45 | 1.82 | |
| succinate | 1.20 | 1.51 | |
| fumarate | 1.38 | 2.07 | |
| malate | 1.28 | 1.91 | |
| Oxidative Phosphorylation | phosphate | 0.90 | 0.98 |
| Sub Pathway | Biochemical Name | Plasma | BF |
|---|---|---|---|
| Fatty Acid Synthesis | malonylcarnitine | n.d. | 1.86 |
| Medium chain fatty acids | caprate (10:0) | 0.73 | n.d. |
| laurate (12:0) | 1.00 | n.d. | |
| Long Chain Fatty Acid | myristate (14:0) | 2.34 | n.d. |
| palmitate (16:0) | 3.16 | n.d. | |
| stearate (18:0) | 2.30 | n.d. | |
| arachidate (20:0) | 1.81 | n.d. | |
| Poly unsaturated fatty acids (n3 and n6) | eicosapentaenoate (EPA; 20:5n3) | 4.49 | n.d. |
| docosapentaenoate (n3 DPA; 22:5n3) | 8.57 | n.d. | |
| docosahexaenoate (DHA; 22:6n3) | 5.69 | n.d. | |
| arachidonate (20:4n6) | 3.48 | n.d. | |
| docosapentaenoate (n6 DPA; 22:5n6) | 2.82 | n.d. | |
| Fatty Acid, Amide | palmitic amide | 0.30 | n.d. |
| oleamide | 0.32 | n.d. | |
| Fatty Acid Metabolism (Acyl Carnitine) | acetylcarnitine (C2) | 1.66 | 1.87 |
| 3-hydroxybutyrylcarnitine (1) | 4.12 | 5.05 | |
| hexanoylcarnitine (C6) | 1.43 | 2.25 | |
| octanoylcarnitine (C8) | 2.14 | 1.89 | |
| laurylcarnitine (C12) | 1.94 | 1.86 | |
| myristoylcarnitine (C14) | 1.80 | n.d. | |
| palmitoylcarnitine (C16) | 1.88 | n.d. | |
| stearoylcarnitine (C18) | 1.82 | n.d. | |
| linoleoylcarnitine (C18:2) | 3.26 | n.d. | |
| arachidonoylcarnitine (C20:4) | 2.37 | n.d. | |
| Carnitine Metabolism | deoxycarnitine | 1.56 | 1.66 |
| carnitine | 1.05 | 1.19 | |
| Ketone Bodies | 3-hydroxybutyrate (BHBA) | 4.44 | 2.97 |
| Eicosanoid | prostaglandin E2 | n.d. | 1.84 |
| prostaglandin A2 | n.d. | 1.83 | |
| prostaglandin F2alpha | n.d. | 1.60 | |
| 12-HETE | 0.85 | n.d. | |
| Phospholipid Metabolism | choline | 1.04 | 1.10 |
| choline phosphate | 0.41 | 0.33 | |
| 1-palmitoyl-2-docosahexaenoyl-GPE (16:0/22:6) | 3.67 | n.d. | |
| 1-stearoyl-2-linoleoyl-GPE (18:0/18:2) | 1.60 | n.d. | |
| 1-stearoyl-2-arachidonoyl-GPE (18:0/20:4) | 1.72 | n.d. | |
| 1-stearoyl-2-docosahexaenoyl-GPE (18:0/22:6) | 11.63 | n.d. | |
| Phosphatidylinositol (PI) | 1-stearoyl-2-arachidonoyl-GPI (18:0/20:4) | 1.95 | n.d. |
| Phosphatidylcholine (PC) | 1-palmitoyl-2-linoleoyl-GPC (16:0/18:2) | 1.60 | 0.80 |
| 1-palmitoyl-2-arachidonoyl-GPC (16:0/20:4n6) | 1.65 | n.d. | |
| 1-stearoyl-2-arachidonoyl-GPC (18:0/20:4) | 1.29 | n.d. | |
| 1-linoleoyl-2-arachidonoyl-GPC (18:2/20:4n6) | 1.64 | n.d. | |
| 1-stearoyl-2-docosahexaenoyl-GPC (18:0/22:6) | 1.90 | n.d. | |
| Lysophospholipid | 1-linoleoyl-GPA (18:2) | 1.69 | n.d. |
| 1-linoleoyl-GPC (18:2) | 1.57 | n.d. | |
| 1-linolenoyl-GPC (18:3) | 2.05 | n.d. | |
| 1-arachidonoyl-GPC (20:4n6) | 1.83 | n.d. | |
| 1-palmitoyl-GPE (16:0) | 1.44 | n.d. | |
| 1-stearoyl-GPE (18:0) | 1.79 | n.d. | |
| 1-linoleoyl-GPE (18:2) | 2.14 | n.d. | |
| 1-arachidonoyl-GPE (20:4n6) | 1.92 | n.d. | |
| 1-stearoyl-GPS (18:0) | 0.31 | n.d. | |
| 1-palmitoyl-GPG (16:0) | 1.51 | n.d. | |
| Plasmalogen | 1-(1-enyl-palmitoyl)-2-oleoyl-GPE (P-16:0/18:1) | 0.37 | n.d. |
| 1-(1-enyl-palmitoyl)-2-linoleoyl-GPE (P-16:0/18:2) | 0.56 | n.d. | |
| 1-(1-enyl-palmitoyl)-2-arachidonoyl-GPE (P-16:0/20:4) | 0.39 | n.d. | |
| 1-(1-enyl-palmitoyl)-2-oleoyl-GPC (P-16:0/18:1) | 0.63 | n.d. | |
| 1-(1-enyl-palmitoyl)-2-linoleoyl-GPC (P-16:0/18:2) | 0.74 | n.d. | |
| Glycerolipid Metabolism | glycerol | 1.44 | n.d. |
| Diacylglycerol | linoleoyl-linolenoyl-glycerol (18:2/18:3) | 48.24 | n.d. |
| Sphingolipid Metabolism | sphinganine | 0.38 | n.d. |
| sphinganine-1-phosphate | 0.81 | n.d. | |
| Mevalonate Metabolism | mevalonate | 1.26 | 1.45 |
| Sterol | cholesterol | 1.69 | n.d. |
| Corticosteroids | cortisol | 1.23 | n.d. |
| Primary Bile Acid Metabolism | cholate | 0.62 | n.d. |
| glycocholate | 1.96 | n.d. | |
| Secondary Bile Acid Metabolism | deoxycholate | 0.62 | 0.59 |
| Sub Pathway | Biochemical Name | Plasma | BF |
|---|---|---|---|
| Glycine, Serine and Threonine Metabolism | glycine | 0.69 | 0.90 |
| N-acetylglycine | 1.47 | 1.15 | |
| serine | 0.87 | 1.02 | |
| N-acetylserine | 1.51 | 0.87 | |
| threonine | 1.16 | 1.17 | |
| N-acetylthreonine | 1.90 | 1.32 | |
| Alanine and Aspartate Metabolism | alanine | 1.41 | 2.01 |
| N-acetylalanine | 1.48 | 1.41 | |
| aspartate | 0.74 | 1.60 | |
| N-acetylaspartate (NAA) | 0.66 | 0.83 | |
| asparagine | 0.99 | 0.91 | |
| N-acetylasparagine | 1.15 | 1.19 | |
| Glutamate Metabolism | glutamate | 0.94 | 2.20 |
| glutamine | 0.68 | 1.97 | |
| N-acetylglutamate | 1.13 | 1.59 | |
| N-acetylglutamine | 1.11 | 1.87 | |
| Histidine Metabolism | histidine | 0.72 | 0.90 |
| 1-methylhistidine | 0.70 | 1.27 | |
| 3-methylhistidine | 1.03 | 1.44 | |
| N-acetylhistidine | 1.07 | n.d. | |
| histamine | 0.18 | n.d. | |
| Lysine Metabolism | lysine | 0.81 | 1.02 |
| N6-acetyllysine | 1.54 | 1.86 | |
| N2,N6-diacetyllysine | 0.50 | n.d. | |
| N6-formyllysine | 2.74 | 2.31 | |
| 2-aminoadipate | 1.61 | 0.61 | |
| Phenylalanine Metabolism | phenylalanine | 0.84 | 0.94 |
| N-acetylphenylalanine | 0.36 | 1.28 | |
| phenylpyruvate | 0.97 | 0.36 | |
| Tyrosine Metabolism | tyrosine | 0.59 | 0.73 |
| N-acetyltyrosine | 0.44 | 1.08 | |
| thyroxine | 1.02 | n.d. | |
| Tryptophan Metabolism | tryptophan | 0.91 | 1.21 |
| N-acetyltryptophan | 0.33 | 1.54 | |
| serotonin | 0.20 | n.d. | |
| Leucine, Isoleucine and Valine Metabolism | leucine | 1.66 | 2.20 |
| N-acetylleucine | 1.37 | 1.97 | |
| isoleucine | 1.57 | 2.04 | |
| N-acetylisoleucine | 1.24 | n.d. | |
| valine | 1.76 | 2.39 | |
| N-acetylvaline | 1.95 | 1.75 | |
| 3-methyl-2-oxobutyrate | 1.64 | 2.52 | |
| 2-hydroxy-3-methylvalerate | 4.95 | 3.83 | |
| Methionine, Cysteine, SAM and Taurine Metabolism | methionine | 1.03 | 1.21 |
| N-acetylmethionine | 1.24 | 1.28 | |
| hypotaurine | 0.35 | 1.55 | |
| Urea cycle; Arginine and Proline Metabolism | arginine | 0.86 | 1.14 |
| proline | 1.61 | 0.97 | |
| N-acetylproline | 2.55 | n.d. | |
| Creatine Metabolism | guanidinoacetate | 2.23 | 2.11 |
| creatine | 1.31 | 1.33 | |
| creatinine | 0.98 | 1.24 | |
| creatine phosphate | 0.29 | n.d. | |
| Glutathione Metabolism | glutathione, oxidized (GSSG) | n.d. | 0.92 |
| Sub Pathway | Biochemical Name | Plasma | BF |
|---|---|---|---|
| Purine Metabolism, (Hypo)Xanthine/Inosine containing | inosine | 0.75 | 0.83 |
| urate | 1.69 | 2.80 | |
| allantoin | 1.00 | 1.76 | |
| Purine Metabolism, Adenine containing | adenosine 5’-diphosphate (ADP) | 0.60 | n.d. |
| adenosine 5’-monophosphate (AMP) | 0.93 | 0.49 | |
| adenosine 3’,5’-cyclic monophosphate (cAMP) | 1.24 | 1.21 | |
| adenosine | 1.28 | 0.70 | |
| adenine | 0.72 | 0.74 | |
| Purine Metabolism, Guanine containing | guanosine-3’,5’-cyclic monophosphate (cGMP) | 1.39 | 1.09 |
| guanosine | 0.94 | 1.13 | |
| guanine | 1.28 | 1.32 | |
| Pyrimidine Metabolism, Uracil containing | uridine | 0.87 | 0.96 |
| uracil | 1.19 | 0.91 | |
| beta-alanine | 1.56 | 1.49 | |
| Pyrimidine Metabolism, Cytidine containing | cytidine | 1.09 | 0.90 |
| cytosine | 1.12 | 1.16 | |
| Pyrimidine Metabolism, Thymine containing | thymidine | 1.15 | 1.05 |
| thymine | 0.94 | 0.98 |
| Sub Pathway | Biochemical Name | Plasma | BF |
|---|---|---|---|
| Nicotinate and Nicotinamide Metabolism | nicotinamide | 0.91 | 0.80 |
| Riboflavin Metabolism | flavin adenine dinucleotide (FAD) | 0.70 | n.d. |
| Pantothenate and CoA Metabolism | pantothenate | 1.28 | 0.84 |
| Tocopherol Metabolism | alpha-tocopherol | 1.46 | n.d. |
| Biotin Metabolism | biotin | n.d. | 0.51 |
| Pterin Metabolism | pterin | n.d. | 0.83 |
| Hemoglobin and Porphyrin Metabolism | bilirubin (Z,Z) | 2.69 | n.d. |
| Thiamine Metabolism | thiamin (Vitamin B1) | 0.65 | n.d. |
| Vitamin A Metabolism | retinol (Vitamin A) | 1.65 | n.d. |
| Vitamin B6 Metabolism | pyridoxamine | 0.46 | n.d. |
| pyridoxal | 1.04 | 0.44 |
| Blastocysts at Day 6 p.c. | n | Diameter Mean ± SD (mm) | Volume Mean (µL) | Gastrulation Stage 1/2 |
|---|---|---|---|---|
| Non-diabetic | 40 | 4.147 ± 0.080 | 26.57 ± 1.47 | 17/19 |
| Diabetic | 40 | 4.076 ± 0.126 | 26.03 ± 1.27 | 18/18 |
| p-value | 0.49 | 0.77 | 0.58 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schindler, M.; Pendzialek, S.M.; Grybel, K.; Seeling, T.; Navarrete Santos, A. Metabolic Profiling in Blastocoel Fluid and Blood Plasma of Diabetic Rabbits. Int. J. Mol. Sci. 2020, 21, 919. https://doi.org/10.3390/ijms21030919
Schindler M, Pendzialek SM, Grybel K, Seeling T, Navarrete Santos A. Metabolic Profiling in Blastocoel Fluid and Blood Plasma of Diabetic Rabbits. International Journal of Molecular Sciences. 2020; 21(3):919. https://doi.org/10.3390/ijms21030919
Chicago/Turabian StyleSchindler, Maria, Sophia Mareike Pendzialek, Katarzyna Grybel, Tom Seeling, and Anne Navarrete Santos. 2020. "Metabolic Profiling in Blastocoel Fluid and Blood Plasma of Diabetic Rabbits" International Journal of Molecular Sciences 21, no. 3: 919. https://doi.org/10.3390/ijms21030919
APA StyleSchindler, M., Pendzialek, S. M., Grybel, K., Seeling, T., & Navarrete Santos, A. (2020). Metabolic Profiling in Blastocoel Fluid and Blood Plasma of Diabetic Rabbits. International Journal of Molecular Sciences, 21(3), 919. https://doi.org/10.3390/ijms21030919