Therapeutic Efficiency of Multiple Applications of Magnetic Hyperthermia Technique in Glioblastoma Using Aminosilane Coated Iron Oxide Nanoparticles: In Vitro and In Vivo Study
Abstract
:1. Introduction
2. Results
2.1. Evaluation of the SPIONa Specific Absortion Rate
2.2. In Vitro Study
2.2.1. Bioluminescence Signal Kinetics of C6 Cell Line Transduced with Luciferase
2.2.2. Evaluation of the SPIONa Internalization into C6 Cells
2.2.3. Evaluation of in Vitro MHT as a Function of Multiple Applications
2.3. In Vivo Study
2.3.1. Evaluation of Tumoral Growth by Histological Analysis
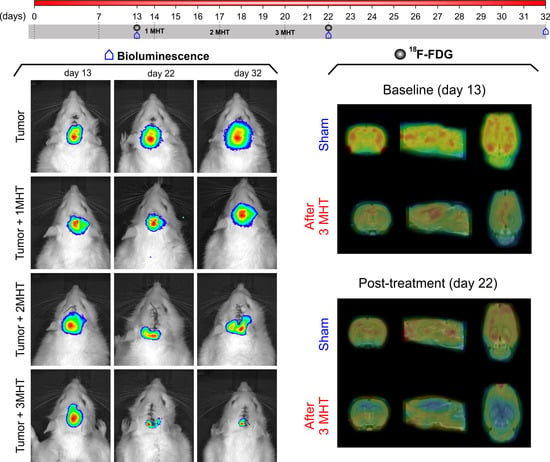
2.3.2. Evaluation of MHT Multiple Applications in Vivo
2.3.3. Spontaneous Locomotion Evaluation in Multiple MHT Applications
3. Discussion
4. Materials and Methods
4.1. Magnetic Nanoparticles
4.2. Evaluation of the Heating Potential of SPIONa
4.3. In Vitro Study
4.3.1. C6 Cells Culture
4.3.2. Kinetics of Bioluminescent Signal of Luciferase Transduced C6 Cells
4.3.3. Labeling C6 Cells with SPIONa and Internalization Imagining
4.3.4. In Vitro MHT
4.4. In Vivo Study
4.4.1. Glioblastoma Tumor Inducion in Animal Model
4.4.2. Evaluation of Tumor Growth by Histological Analysis
4.4.3. In Vivo Experimental Design of Magnetic Hyperthermia
In Vivo MHT Therapeutic Process
Therapeutic Process Evaluation by BLI
Evaluation of MHT Therapeutic Effect by 18F-FDG-PET
Evaluation of MHT Therapeutic Effect by Spontaneous Locomotor Activity
4.5. Statistic Analysis
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| MHT | Magnetic hyperthermia |
| GBM | Glioblastoma |
| SPIONa | SPION coated with aminosilane |
| SPION | Superparamagnetic iron oxide nanoparticle |
| SAR | Specific absorption rate |
| BLI | Bioluminescence |
| PET | Positron emission tomography |
| TMZ | Temozolomide |
| MNP | Magnetic nanoparticles |
| AMF | Alternating magnetic field |
| MRI | Magnetic resonance imaging |
| NIRF | Near-infrared fluorescence |
| SPECT | Single Photon Emission Computed Tomography |
| MPI | Magnetic particle imaging |
| CT | Computed tomography |
| ATP | Adenosinetriphosphate |
| 2D | Two-dimensional |
| 18F-FDG | 18F-2-fluoro-2-deoxy-D-glucose |
| SD | Standard deviation |
| S-MOV | Slow horizontal movement |
| F-MOV | Fast horizontal movement |
| S-REA | Slow rearing |
| F-REA | Fast rearing |
| DH | Hydrodynamic diameter |
| ILP | Intrinsic loss power |
| PEI | Polyethyleneimine |
| Hsp | Heat shock protein |
| MCL | Magnetite cationic liposomes |
| DMEM/F12 | Dulbecco’s modified eagle’s medium: nutrient mixture F-12 |
| FBS | Fetal bovine serum |
| PBS | Phosphate buffered saline |
| PFA | Paraformaldehyde |
| IR | Infrared |
Appendix A
| SAR Value Obtained for Frequency and Magnetic Field Combinations | Mean Difference | Standard Error | p Bonferroni | |
|---|---|---|---|---|
| 309 kHz–100 G | 309 kHz–200 G | −50.968 | 4.637 | <0.001 |
| 309 kHz–300 G | −165.508 | 4.637 | <0.001 | |
| 364 kHz–100 G | −2.997 | 4.637 | 1.000 | |
| 364 kHz–200 G | −58.007 | 4.637 | <0.001 | |
| 364 kHz–300 G | −181.675 | 4.637 | <0.001 | |
| 420 kHz–100 G | −4.506 | 4.637 | 1.000 | |
| 420 kHz–200 G | −75.329 | 4.637 | <0.001 | |
| 420 kHz–300 G | −234.986 | 4.637 | <0.001 | |
| 557 kHz–100 G | −7.290 | 4.637 | 1.000 | |
| 557 kHz–200 G | −78.983 | 4.637 | <0.001 | |
| 557 kHz–300 G | −316.281 | 4.637 | <0.001 | |
| 874 kHz–100 G | −316.281 | 4.637 | 0.542 | |
| 874 kHz–200 G | −316.281 | 4.637 | <0.001 | |
| 309 kHz–200 G | 309 kHz–300 G | −114.540 | 4.637 | <0.001 |
| 364 kHz–100 G | 47.972 | 4.637 | <0.001 | |
| 364 kHz–200 G | −7.039 | 4.637 | 1.000 | |
| 364 kHz–300 G | −130.707 | 4.637 | <0.001 | |
| 420 kHz–100 G | 46.462 | 4.637 | <0.001 | |
| 420 kHz–200 G | −24.360 | 4.637 | <0.001 | |
| 420 kHz–300 G | −184.018 | 4.637 | <0.001 | |
| 557 kHz–100 G | 43.679 | 4.637 | <0.001 | |
| 557 kHz–200 G | −28.015 | 4.637 | <0.001 | |
| 557 kHz–300 G | −265.313 | 4.637 | <0.001 | |
| 874 kHz–100 G | −265.313 | 4.637 | <0.001 | |
| 874 kHz–200 G | −265.313 | 4.637 | <0.001 | |
| 309 kHz–300 G | 364 kHz–100 G | 162.511 | 4.637 | <0.001 |
| 364 kHz–200 G | 107.501 | 4.637 | <0.001 | |
| 364 kHz–300 G | −16.167 | 4.637 | 0.087 | |
| 420 kHz–100 G | 161.001 | 4.637 | <0.001 | |
| 420 kHz–200 G | 90.179 | 4.637 | <0.001 | |
| 420 kHz–300 G | −69.478 | 4.637 | <0.001 | |
| 557 kHz–100 G | 158.218 | 4.637 | <0.001 | |
| 557 kHz–200 G | 86.525 | 4.637 | <0.001 | |
| 557 kHz–300 G | −150.773 | 4.637 | <0.001 | |
| 874 kHz–100 G | −150.773 | 4.637 | <0.001 | |
| 874 kHz–200 G | −150.773 | 4.637 | 1.000 | |
| 364 kHz–100 G | 364 kHz–200 G | −55.011 | 4.637 | <0.001 |
| 364 kHz–300 G | −178.678 | 4.637 | <0.001 | |
| 420 kHz–100 G | −1.510 | 4.637 | 1.000 | |
| 420 kHz–200 G | −72.332 | 4.637 | <0.001 | |
| 420 kHz–300 G | −231.990 | 4.637 | <0.001 | |
| 557 kHz–100 G | −4.293 | 4.637 | 1.000 | |
| 557 kHz–200 G | −75.986 | 4.637 | <0.001 | |
| 557 kHz–300 G | −313.284 | 4.637 | <0.001 | |
| 874 kHz–100 G | −313.284 | 4.637 | 1.000 | |
| 874 kHz–200 G | −313.284 | 4.637 | <0.001 | |
| 364 kHz–200 G | 364 kHz–300 G | −123.668 | 4.637 | <0.001 |
| 420 kHz–100 G | 53.501 | 4.637 | <0.001 | |
| 420 kHz–200 G | −17.321 | 4.637 | 0.040 | |
| 420 kHz–300 G | −176.979 | 4.637 | <0.001 | |
| 557 kHz–100 G | 50.718 | 4.637 | <0.001 | |
| 557 kHz–200 G | −20.976 | 4.637 | 0.003 | |
| 557 kHz–300 G | −258.274 | 4.637 | <0.001 | |
| 874 kHz–100 G | −258.274 | 4.637 | <0.001 | |
| 874 kHz–200 G | −258.274 | 4.637 | <0.001 | |
| 364 kHz–300 G | 420 kHz–100 G | 177.169 | 4.637 | <0.001 |
| 420 kHz–200 G | 106.346 | 4.637 | <0.001 | |
| 420 kHz–300 G | −53.311 | 4.637 | <0.001 | |
| 557 kHz–100 G | 174.385 | 4.637 | <0.001 | |
| 557 kHz–200 G | 102.692 | 4.637 | <0.001 | |
| 557 kHz–300 G | −134.606 | 4.637 | <0.001 | |
| 874 kHz–100 G | −134.606 | 4.637 | <0.001 | |
| 874 kHz–200 G | −134.606 | 4.637 | 1.000 | |
| 420 kHz–100 G | 420 kHz–200 G | −70.822 | 4.637 | <0.001 |
| 420 kHz–300 G | −230.480 | 4.637 | <0.001 | |
| 557 kHz–100 G | −2.783 | 4.637 | 1.000 | |
| 557 kHz–200 G | −74.477 | 4.637 | <0.001 | |
| 557 kHz–300 G | −311.775 | 4.637 | <0.001 | |
| 874 kHz–100 G | −311.775 | 4.637 | 1.000 | |
| 874 kHz–200 G | −311.775 | 4.637 | <0.001 | |
| 420 kHz–200 G | 420 kHz–300 G | −159.658 | 4.637 | <0.001 |
| 557 kHz–100 G | 68.039 | 4.637 | <0.001 | |
| 557 kHz–200 G | −3.654 | 4.637 | 1.000 | |
| 557 kHz–300 G | −240.952 | 4.637 | <0.001 | |
| 874 kHz–100 G | −240.952 | 4.637 | <0.001 | |
| 874 kHz–200 G | −240.952 | 4.637 | <0.001 | |
| 420 kHz–300 G | 557 kHz–100 G | 227.697 | 4.637 | <0.001 |
| 557 kHz–200 G | 156.003 | 4.637 | <0.001 | |
| 557 kHz–300 G | −81.295 | 4.637 | <0.001 | |
| 874 kHz–100 G | −81.295 | 4.637 | <0.001 | |
| 874 kHz–200 G | −81.295 | 4.637 | <0.001 | |
| 557 kHz–100 G | 557 kHz–200 G | −71.693 | 4.637 | <0.001 |
| 557 kHz–300 G | −308.991 | 4.637 | <0.001 | |
| 874 kHz–100 G | −308.991 | 4.637 | 1.000 | |
| 874 kHz–200 G | −308.991 | 4.637 | <0.001 | |
| 557 kHz–200 G | 557 kHz–300 G | −237.298 | 4.637 | <0.001 |
| 874 kHz–100 G | −237.298 | 4.637 | <0.001 | |
| 874 kHz–200 G | −237.298 | 4.637 | <0.001 | |
| 557 kHz–300 G | 874 kHz–100 G | −237.298 | 4.637 | <0.001 |
| 874 kHz–200 G | −237.298 | 4.637 | <0.001 | |
| 874 kHz–100 G | 874 kHz–200 G | −237.298 | 4.637 | <0.001 |
| Groups | Mean Difference | 95% IC of Mean Difference | p | ||
|---|---|---|---|---|---|
| Inferior | Superior | ||||
| C6 without SPIONa | C6 with SPIONa | 4.000 × 107 | −4.279 × 108 | 5.079 × 108 | 1.000 |
| C6-f1-B without SPIONa | −2.950 × 108 | −7.629 × 108 | 1.729 × 108 | 0.906 | |
| C6-f1-B with SPIONa | 2.323 × 109 | 1.855 × 109 | 2.790 × 109 | <0.001 | |
| C6-f2-B without SPIONa | −3.050 × 108 | −7.729 × 108 | 1.629 × 108 | 0.794 | |
| C6-f2-B with SPIONa | 3.197 × 109 | 2.729 × 109 | 3.665 × 109 | <0.001 | |
| C6 with SPIONa | C6-f1-B without SPIONa | −3.350 × 108 | −8.029 × 108 | 1.329 × 108 | 0.530 |
| C6-f1-B with SPIONa | 2.283 × 109 | 1.815 × 109 | 2.750 × 109 | <0.001 | |
| C6-f2-B without SPIONa | −3.450 × 108 | −8.129 × 108 | 1.229 × 108 | 0.462 | |
| C6-f2-B with SPIONa | 3.157 × 109 | 2.689 × 109 | 3.625 × 109 | <0.001 | |
| C6-f1-B without SPIONa | C6-f1-B with SPIONa | 2.618 × 109 | 2.150 × 109 | 3.085 × 109 | <0.001 |
| C6-f2-B without SPIONa | −1.000 × 107 | −4.779 × 108 | 4.579 × 108 | 1.000 | |
| C6-f2-B with SPIONa | 3.492 × 109 | 3.024 × 109 | 3.960 × 109 | <0.001 | |
| C6-f1-B with SPIONa | C6-f2-B without SPIONa | −2.628 × 109 | −3.095 × 109 | -2.160 × 109 | <0.001 |
| C6-f2-B with SPIONa | 8.745 × 108 | 4.066 × 108 | 1.342 × 109 | <0.001 | |
| C6-f2-B without SPIONa | C6-f2-B with SPIONa | 3.502 × 109 | 3.034 × 109 | 3.970 × 109 | <0.001 |
| Groups | Mean Difference | 95% IC of Mean Difference | p | ||
|---|---|---|---|---|---|
| Inferior | Superior | ||||
| C6 without SPIONa | C6 with SPIONa | 5.388 × 106 | −6.056 × 108 | 6.164 × 108 | 1.000 |
| C6-f1-B without SPIONa | 5.383 × 106 | −6.056 × 108 | 6.164 × 108 | 1.000 | |
| C6-f1-B with SPIONa | 3.933 × 109 | 3.322 × 109 | 4.544 × 109 | <0.001 | |
| C6-f2-B without SPIONa | 5.388 × 106 | −6.056 × 108 | 6.164 × 108 | 1.000 | |
| C6-f2-B with SPIONa | 4.588 × 109 | 3.977 × 109 | 5.199 × 109 | <0.001 | |
| C6 with SPIONa | C6-f1-B without SPIONa | −4656.000 | −6.110 × 108 | 6.110 × 108 | 1.000 |
| C6-f1-B with SPIONa | 3.928 × 109 | 3.317 × 109 | 4.539 × 109 | <0.001 | |
| C6-f2-B without SPIONa | 0.000 | −6.110 × 108 | 6.110 × 108 | 1.000 | |
| C6-f2-B with SPIONa | 4.582 × 109 | 3.971 × 109 | 5.193 × 109 | <0.001 | |
| C6-f1-B without SPIONa | C6-f1-B with SPIONa | 3.928 × 109 | 3.317 × 109 | 4.539 × 109 | <0.001 |
| C6-f2-B without SPIONa | 4656.000 | −6.110 × 108 | 6.110 × 108 | 1.000 | |
| C6-f2-B with SPIONa | 4.582 × 109 | 3.971 × 109 | 5.193 × 109 | <0.001 | |
| C6-f1-B with SPIONa | C6-f2-B without SPIONa | −3.928 × 109 | −4.539 × 109 | −3.317 × 109 | <0.001 |
| C6-f2-B with SPIONa | 6.546 × 108 | 4.360 × 107 | 1.266 × 109 | 0.047 | |
| C6-f2-B without SPIONa | C6-f2-B with SPIONa | 4.582 × 109 | 3.971 × 109 | 5.193 × 109 | <0.001 |
| Groups | Mean Difference | 95% IC of Mean Difference | p | ||
|---|---|---|---|---|---|
| Inferior | Superior | ||||
| C6 without SPIONa | C6 with SPIONa | −8.728 × 107 | −7.977 × 108 | 6.231 × 108 | 1.000 |
| C6-f1-B without SPIONa | −2.182 × 108 | −9.286 × 108 | 4.922 × 108 | 1.000 | |
| C6-f1-B with SPIONa | 5.979 × 109 | 5.268 × 109 | 6.689 × 109 | <0.001 | |
| C6-f2-B without SPIONa | −1.746 × 108 | −8.850 × 108 | 5.358 × 108 | 1.000 | |
| C6-f2-B with SPIONa | 5.455 × 109 | 4.745 × 109 | 6.165 × 109 | <0.001 | |
| C6 with SPIONa | C6-f1-B without SPIONa | −1.309 × 108 | −8.413 × 108 | 5.795 × 108 | 1.000 |
| C6-f1-B with SPIONa | 6.066 × 109 | 5.356 × 109 | 6.776 × 109 | <0.001 | |
| C6-f2-B without SPIONa | −8.729 × 107 | −7.977 × 108 | 6.231 × 108 | 1.000 | |
| C6-f2-B with SPIONa | 5.542 × 109 | 4.832 × 109 | 6.253 × 109 | <0.001 | |
| C6-f1-B without SPIONa | C6-f1-B with SPIONa | 6.197 × 109 | 5.486 × 109 | 6.907 × 109 | <0.001 |
| C6-f2-B without SPIONa | 4.363 × 107 | −6.668 × 108 | 7.540 × 108 | 1.000 | |
| C6-f2-B with SPIONa | 5.673 × 109 | 4.963 × 109 | 6.384 × 109 | <0.001 | |
| C6-f1-B with SPIONa | C6-f2-B without SPIONa | −6.153 × 109 | −6.864 × 109 | -5.443 × 109 | <0.001 |
| C6-f2-B with SPIONa | −5.237 × 108 | −1.234 × 109 | 1.867 × 108 | 0.463 | |
| C6-f2-B without SPIONa | C6-f2-B with SPIONa | 5.630 × 109 | 4.919 × 109 | 6.340 × 109 | <0.001 |
References
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pisapia, D.J. The Updated World Health Organization Glioma Classification: Cellular and Molecular Origins of Adult Infiltrating Gliomas. Arch. Pathol. Lab. Med. 2017, 141, 1633–1645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ostrom, Q.T.; Gittleman, H.; Truitt, G.; Boscia, A.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2011–2015. Neuro-Oncol. 2018, 20, iv1–iv86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Braun, K.; Ahluwalia, M.S. Treatment of Glioblastoma in Older Adults. Curr. Oncol. Rep. 2017, 19, 81. [Google Scholar] [CrossRef]
- Spirou, S.V.; Basini, M.; Lascialfari, A.; Sangregorio, C.; Innocenti, C. Magnetic Hyperthermia and Radiation Therapy: Radiobiological Principles and Current Practice (dagger). Nanomaterials 2018, 8, 401. [Google Scholar] [CrossRef] [Green Version]
- Dadfar, S.M.; Roemhild, K.; Drude, N.I.; von Stillfried, S.; Knüchel, R.; Kiessling, F.; Lammers, T. Iron oxide nanoparticles: Diagnostic, therapeutic and theranostic applications. Adv. Drug Deliv. Rev. 2019, 138, 302–325. [Google Scholar] [CrossRef]
- Kuchma, E.; Kubrin, S.; Soldatov, A. The Local Atomic Structure of Colloidal Superparamagnetic Iron Oxide Nanoparticles for Theranostics in Oncology. Biomedicines 2018, 6, 78. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.M.; Wang, Y.X.; Leung, K.C.; Lee, S.F.; Zhao, F.; Wang, D.W.; Lai, J.M.; Wan, C.; Cheng, C.H.; Ahuja, A.T. Enhanced cellular uptake of aminosilane-coated superparamagnetic iron oxide nanoparticles in mammalian cell lines. Int. J. Nanomed. 2012, 7, 953–964. [Google Scholar] [CrossRef] [Green Version]
- Noha, S.-H.; Moona, S.H.; Shina, T.-H.; Lima, Y.; Cheona, J. Recent advances of magneto-thermal capabilities of nanoparticles: From design principles to biomedical applications. Nano Today 2017, 13, 61–76. [Google Scholar] [CrossRef]
- Feng, Q.; Liu, Y.; Huang, J.; Chen, K.; Huang, J.; Xiao, K. Uptake, distribution, clearance, and toxicity of iron oxide nanoparticles with different sizes and coatings. Sci. Rep. 2018, 8, 2082. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Yathindranath, V.; Worden, M.; Thliveris, J.A.; Chu, S.; Parkinson, F.E.; Hegmann, T.; Miller, D.W. Characterization of cellular uptake and toxicity of aminosilane-coated iron oxide nanoparticles with different charges in central nervous system-relevant cell culture models. Int. J. Nanomed. 2013, 8, 961–970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, A.; Shinkai, M.; Honda, H.; Yoshikawa, K.; Saga, S.; Wakabayashi, T.; Yoshida, J.; Kobayashi, T. Heat shock protein 70 expression induces antitumor immunity during intracellular hyperthermia using magnetite nanoparticles. Cancer Immunol. Immunother. 2003, 52, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Yanase, M.; Shinkai, M.; Honda, H.; Wakabayashi, T.; Yoshida, J.; Kobayashi, T. Intracellular hyperthermia for cancer using magnetite cationic liposomes: Ex vivo study. Jpn. J. Cancer Res. 1997, 88, 630–632. [Google Scholar] [CrossRef]
- Yanase, M.; Shinkai, M.; Honda, H.; Wakabayashi, T.; Yoshida, J.; Kobayashi, T. Antitumor immunity induction by intracellular hyperthermia using magnetite cationic liposomes. Jpn. J. Cancer Res. 1998, 89, 775–782. [Google Scholar] [CrossRef]
- Rabias, I.; Tsitrouli, D.; Karakosta, E.; Kehagias, T.; Diamantopoulos, G.; Fardis, M.; Stamopoulos, D.; Maris, T.G.; Falaras, P.; Zouridakis, N.; et al. Rapid magnetic heating treatment by highly charged maghemite nanoparticles on Wistar rats exocranial glioma tumors at microliter volume. Biomicrofluidics 2010, 4. [Google Scholar] [CrossRef] [Green Version]
- Ito, A.; Shinkai, M.; Honda, H.; Kobayashi, T. Heat-inducible TNF-alpha gene therapy combined with hyperthermia using magnetic nanoparticles as a novel tumor-targeted therapy. Cancer Gene Ther. 2001, 8, 649–654. [Google Scholar] [CrossRef] [Green Version]
- Van Landeghem, F.K.; Maier-Hauff, K.; Jordan, A.; Hoffmann, K.T.; Gneveckow, U.; Scholz, R.; Thiesen, B.; Bruck, W.; von Deimling, A. Post-mortem studies in glioblastoma patients treated with thermotherapy using magnetic nanoparticles. Biomaterials 2009, 30, 52–57. [Google Scholar] [CrossRef]
- Gupta, R.; Sharma, D. Biofunctionalization of magnetite nanoparticles with stevioside: Effect on the size and thermal behaviour for use in hyperthermia applications. Int. J. Hyperth. 2019. [Google Scholar] [CrossRef] [Green Version]
- Maier-Hauff, K.; Rothe, R.; Scholz, R.; Gneveckow, U.; Wust, P.; Thiesen, B.; Feussner, A.; von Deimling, A.; Waldoefner, N.; Felix, R.; et al. Intracranial thermotherapy using magnetic nanoparticles combined with external beam radiotherapy: Results of a feasibility study on patients with glioblastoma multiforme. J. Neurooncol. 2007, 81, 53–60. [Google Scholar] [CrossRef]
- Maier-Hauff, K.; Ulrich, F.; Nestler, D.; Niehoff, H.; Wust, P.; Thiesen, B.; Orawa, H.; Budach, V.; Jordan, A. Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. J. Neurooncol. 2011, 103, 317–324. [Google Scholar] [CrossRef] [Green Version]
- Alphandéry, E.; Idbaih, A.; Adam, C.; Delattre, J.Y.; Schmitt, C.; Guyot, F.; Chebbi, I. Development of non-pyrogenic magnetosome minerals coated with poly-l-lysine leading to full disappearance of intracranial U87-Luc glioblastoma in 100% of treated mice using magnetic hyperthermia. Biomaterials 2017, 141, 210–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsiapa, I.; Efthimiadou, E.K.; Fragogeorgi, E.; Loudos, G.; Varvarigou, A.D.; Bouziotis, P.; Kordas, G.C.; Mihailidis, D.; Nikiforidis, G.C.; Xanthopoulos, S.; et al. 99mTc-labeled aminosilane-coated iron oxide nanoparticles for molecular imaging of ανβ3-mediated tumor expression and feasibility for hyperthermia treatment. J. Colloid Interface Sci. 2014, 433, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Alphandéry, E.; Idbaih, A.; Adam, C.; Delattre, J.Y.; Schmitt, C.; Guyot, F.; Chebbi, I. Chains of magnetosomes with controlled endotoxin release and partial tumor occupation induce full destruction of intracranial U87-Luc glioma in mice under the application of an alternating magnetic field. J. Control. Release 2017, 262, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Lu, I.L.; Chiang, W.H.; Lin, Y.W.; Tsai, Y.C.; Chen, H.H.; Chang, C.W.; Chiang, C.S.; Chiu, H.C. Tumortropic adipose-derived stem cells carrying smart nanotherapeutics for targeted delivery and dual-modality therapy of orthotopic glioblastoma. J. Control. Release 2017, 254, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Muroski, M.E.; Petit, D.C.M.C.; Mansell, R.; Vemulkar, T.; Morshed, R.A.; Han, Y.; Balyasnikova, I.V.; Horbinski, C.M.; Huang, X.; et al. Rotating magnetic field induced oscillation of magnetic particles for in vivo mechanical destruction of malignant glioma. J. Control. Release 2016, 223, 75–84. [Google Scholar] [CrossRef] [Green Version]
- Coisson, M.; Barrera, G.; Celegato, F.; Martino, L.; Kane, S.N.; Raghuvanshi, S.; Vinai, F.; Tiberto, P. Hysteresis losses and specific absorption rate measurements in magnetic nanoparticles for hyperthermia applications. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 1545–1558. [Google Scholar] [CrossRef]
- Zamora-Mora, V.; Fernández-Gutiérrez, M.; González-Gómez, Á.; Sanz, B.; Román, J.S.; Goya, G.F.; Hernández, R.; Mijangos, C. Chitosan nanoparticles for combined drug delivery and magnetic hyperthermia: From preparation to in vitro studies. Carbohydr. Polym. 2017, 157, 361–370. [Google Scholar] [CrossRef] [Green Version]
- Pala, K.; Serwotka, A.; Jeleń, F.; Jakimowicz, P.; Otlewski, J. Tumor-specifc hyperthermia with aptamer-tagged superparamagnetic nanoparticles. Int. J. Nanomed. 2013, 9, 67–76. [Google Scholar] [CrossRef] [Green Version]
- Tapeinos, C.; Marino, A.; Battaglini, M.; Migliorin, S.; Brescia, R.; Scarpellini, A.; De Julián Fernández, C.; Prato, M.; Drago, F.; Ciofani, G. Stimuli-responsive lipid-based magnetic nanovectors increase apoptosis in glioblastoma cells through synergic intracellular hyperthermia and chemotherapy. Nanoscale 2019, 11, 72–88. [Google Scholar] [CrossRef] [Green Version]
- Huszthy, P.C.; Daphu, I.; Niclou, S.P.; Stieber, D.; Nigro, J.M.; Sakariassen, P.Ø.; Miletic, H.; Thorsen, F.; Bjerkvig, R. In vivo models of primary brain tumors: Pitfalls and perspectives. Neuro-Oncol. 2012, 14, 979–993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Sol-Fernández, S.; Portilla-Tundidor, Y.; Gutiérrez, L.; Odio, O.F.; Reguera, E.; Barber, D.F.; Morales, M.P. Flower-like Mn-Doped Magnetic Nanoparticles Functionalized with αvβ3-Integrin-Ligand to Efficiently Induce Intracellular Heat after Alternating Magnetic Field Exposition, Triggering Glioma Cell Death. ACS Appl. Mater. Interfaces 2019, 11, 26648–26663. [Google Scholar] [CrossRef] [PubMed]
- Herynek, V.; Turnovcová, K.; Veverka, P.; Dědourková, T.; Žvátora, P.; Jendelová, P.; Gálisová, A.; Kosinová, L.; Jiráková, K.; Syková, E. Using ferromagnetic nanoparticles with low curie temperature for magnetic resonance imaging-guided thermoablation. Int. J. Nanomed. 2016, 11, 3801–3811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, V.M.; Huynh, E.; Tang, S.; Uskoković, V. Brain and bone cancer targeting by a ferrofluid composed of superparamagnetic iron-oxide/silica/carbon nanoparticles (earthicles). Acta Biomater. 2019, 88, 422–447. [Google Scholar] [CrossRef]
- Pernal, S.; Wu, V.M.; Uskoković, V. Hydroxyapatite as a Vehicle for the Selective Effect of Superparamagnetic Iron Oxide Nanoparticles against Human Glioblastoma Cells. ACS Appl. Mater. Interfaces 2017, 9, 39283–39302. [Google Scholar] [CrossRef] [PubMed]
- Feuser, P.E.; Bubniak, L.D.S.; Silva, M.C.D.S.; Viegas, A.D.C.; Castilho Fernandes, A.; Ricci-Junior, E.; Nele, M.; Tedesco, A.C.; Sayer, C.; De Araújo, P.H.H. Encapsulation of magnetic nanoparticles in poly(methyl methacrylate) by miniemulsion and evaluation of hyperthermia in U87MG cells. Eur. Polym. J. 2015, 68, 355–365. [Google Scholar] [CrossRef]
- Spirou, S.V.; Costa Lima, S.A.; Bouziotis, P.; Vranjes-Djuric, S.; Efthimiadou, E.; Laurenzana, A.; Barbosa, A.I.; Garcia-Alonso, I.; Jones, C.; Jankovic, D.; et al. Recommendations for In Vitro and In Vivo Testing of Magnetic Nanoparticle Hyperthermia Combined with Radiation Therapy. Nanomaterials 2018, 8, 306. [Google Scholar] [CrossRef] [Green Version]
- Merle, P.; Camus, P.; Abergel, A.; Pageaux, G.P.; Masliah, C.; Bronowicki, J.P.; Zarski, J.P.; Pelletier, G.; Bouattour, M.; Farloux, L.; et al. Safety and efficacy of intra-arterial hepatic chemotherapy with doxorubicin-loaded nanoparticles in hepatocellular carcinoma. ESMO Open 2017, 2, e000238. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.Y.; Wang, Y.X.; Lin, Y.; Zhang, J.S.; Yang, F.; Zhou, Q.L.; Liao, Y.Y. Advance of molecular imaging technology and targeted imaging agent in imaging and therapy. BioMed Res. Int. 2014, 2014, 819324. [Google Scholar] [CrossRef] [Green Version]
- Pi, Z.; Huang, Y.; Shen, Y.; Zeng, X.; Hu, Y.; Chen, T.; Li, C.; Yu, H.; Chen, S.; Chen, X. Sonodynamic Therapy on Intracranial Glioblastoma Xenografts Using Sinoporphyrin Sodium Delivered by Ultrasound with Microbubbles. Ann. Biomed. Eng. 2019, 47, 549–562. [Google Scholar] [CrossRef]
- Carvalho, L.A.; Teng, J.; Fleming, R.L.; Tabet, E.I.; Zinter, M.; de Melo Reis, R.A.; Tannous, B.A. Olfactory Ensheathing Cells: A Trojan Horse for Glioma Gene Therapy. J. Natl. Cancer Inst. 2019, 111, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Guerra-Rebollo, M.; Nogueira de Moraes, C.; Alcoholado, C.; Soler-Botija, C.; Sanchez-Cid, L.; Vila, O.F.; Meca-Cortes, O.; Ramos-Romero, S.; Rubio, N.; Becerra, J.; et al. Glioblastoma Bystander Cell Therapy: Improvements in Treatment and Insights into the Therapy Mechanisms. Mol. Ther. Oncolytics 2018, 11, 39–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meca-Cortes, O.; Guerra-Rebollo, M.; Garrido, C.; Borros, S.; Rubio, N.; Blanco, J. CRISPR/Cas9-Mediated Knockin Application in Cell Therapy: A Non-viral Procedure for Bystander Treatment of Glioma in Mice. Mol. Ther. Nucleic Acids 2017, 8, 395–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Genevois, C.; Loiseau, H.; Couillaud, F. In Vivo Follow-up of Brain Tumor Growth via Bioluminescence Imaging and Fluorescence Tomography. Int. J. Mol. Sci. 2016, 17, 1815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez-Cabezas, S.; Montes-Robles, R.; Gallo, J.; Sancenon, F.; Martinez-Manez, R. Combining magnetic hyperthermia and dual T1/T2 MR imaging using highly versatile iron oxide nanoparticles. Dalton Trans. 2019, 48, 3883–3892. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Hsu, C.H.; Li, Z.; Kim, T.S.; Hwang, L.P.; Lin, Y.C.; Lin, Y.Y. Magnetic resonance nano-theranostics for glioblastoma multiforme. Curr. Pharm. Des. 2015, 21, 5256–5266. [Google Scholar] [CrossRef]
- Milanovic, D.; Braun, F.; Weber, W.; Grosu, A.L.; Behe, M.; Niedermann, G. The influence of the combined treatment with Vadimezan (ASA404) and taxol on the growth of U251 glioblastoma xenografts. BMC Cancer 2012, 12, 242. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Truillet, C.; Flavell, R.R.; Brewer, T.F.; Evans, M.J.; Wilson, D.M.; Chang, C.J. A reactivity-based [(18)F]FDG probe for in vivo formaldehyde imaging using positron emission tomography. Chem. Sci. 2016, 7, 5503–5507. [Google Scholar] [CrossRef] [Green Version]
- Sha, W.; Ye, H.; Iwamoto, K.S.; Wong, K.P.; Wilks, M.Q.; Stout, D.; McBride, W.; Huang, S.C. Factors affecting tumor (18) F-FDG uptake in longitudinal mouse PET studies. EJNMMI Res. 2013, 3, 51. [Google Scholar] [CrossRef] [Green Version]
- Van Dort, M.E.; Rehemtulla, A.; Ross, B.D. PET and SPECT Imaging of Tumor Biology: New Approaches towards Oncology Drug Discovery and Development. Curr. Comput. Aided Drug Des. 2008, 4, 46–53. [Google Scholar] [CrossRef] [Green Version]
- Yu, E.Y.; Bishop, M.; Zheng, B.; Ferguson, R.M.; Khandhar, A.P.; Kemp, S.J.; Krishnan, K.M.; Goodwill, P.W.; Conolly, S.M. Magnetic Particle Imaging: A Novel in Vivo Imaging Platform for Cancer Detection. Nano Lett. 2017, 17, 1648–1654. [Google Scholar] [CrossRef] [PubMed]
- Tay, Z.W.; Chandrasekharan, P.; Chiu-Lam, A.; Hensley, D.W.; Dhavalikar, R.; Zhou, X.Y.; Yu, E.Y.; Goodwill, P.W.; Zheng, B.; Rinaldi, C.; et al. Magnetic Particle Imaging-Guided Heating in Vivo Using Gradient Fields for Arbitrary Localization of Magnetic Hyperthermia Therapy. ACS Nano 2018, 12, 3699–3713. [Google Scholar] [CrossRef] [PubMed]
- Souza, T.K.F.; Nucci, M.P.; Mamani, J.B.; da Silva, H.R.; Fantacini, D.M.C.; de Souza, L.E.B.; Picanco-Castro, V.; Covas, D.T.; Vidoto, E.L.; Tannus, A.; et al. Image and motor behavior for monitoring tumor growth in C6 glioma model. PLoS ONE 2018, 13, e0201453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atif, F.; Yousuf, S.; Espinosa-Garcia, C.; Sergeeva, E.; Stein, D.G. Progesterone Treatment Attenuates Glycolytic Metabolism and Induces Senescence in Glioblastoma. Sci. Rep. 2019, 9, 988. [Google Scholar] [CrossRef] [PubMed]
- Petty, R.D.; Sutherland, L.A.; Hunter, E.M.; Cree, I.A. Comparison of MTT and ATP-based assays for the measurement of viable cell number. J. Biolumin. Chemilumin. 1995, 10, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Magalhaes, C.M.; Esteves da Silva, J.C.; Pinto da Silva, L. Chemiluminescence and Bioluminescence as an Excitation Source in the Photodynamic Therapy of Cancer: A Critical Review. Chemphyschem Eur. J. Chem. Phys. Phys. Chem. 2016, 17, 2286–2294. [Google Scholar] [CrossRef]
- Mezzanotte, L.; van‘t Root, M.; Karatas, H.; Goun, E.A.; Lowik, C. In Vivo Molecular Bioluminescence Imaging: New Tools and Applications. Trends Biotechnol. 2017, 35, 640–652. [Google Scholar] [CrossRef]
- Viel, T.; Talasila, K.M.; Monfared, P.; Wang, J.; Jikeli, J.F.; Waerzeggers, Y.; Neumaier, B.; Backes, H.; Brekka, N.; Thorsen, F.; et al. Analysis of the growth dynamics of angiogenesis-dependent and -independent experimental glioblastomas by multimodal small-animal PET and MRI. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2012, 53, 1135–1145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donche, S.; Verhoeven, J.; Descamps, B.; Bolcaen, J.; Deblaere, K.; Boterberg, T.; Van den Broecke, C.; Vanhove, C.; Goethals, I. The Path Toward PET-Guided Radiation Therapy for Glioblastoma in Laboratory Animals: A Mini Review. Front. Med. 2019, 6, 5. [Google Scholar] [CrossRef] [Green Version]
- Moreau, A.; Febvey, O.; Mognetti, T.; Frappaz, D.; Kryza, D. Contribution of Different Positron Emission Tomography Tracers in Glioma Management: Focus on Glioblastoma. Front. Oncol. 2019, 9, 1134. [Google Scholar] [CrossRef] [Green Version]
- Bolcaen, J.; Acou, M.; Descamps, B.; Kersemans, K.; Deblaere, K.; Vanhove, C.; Goethals, I. PET for Therapy Response Assessment in Glioblastoma. In Glioblastoma; Codon Publications Copyright, De Vleeschouwer, S., Eds.; The Authors: Brisbane, Australia, 2017. [Google Scholar] [CrossRef] [Green Version]
- Verger, A.; Langen, K.J. PET Imaging in Glioblastoma: Use in Clinical Practice. In Glioblastoma; Codon Publications Copyright, De Vleeschouwer, S., Eds.; The Authors: Brisbane, Australia, 2017. [Google Scholar] [CrossRef]
- Aronen, H.J.; Pardo, F.S.; Kennedy, D.N.; Belliveau, J.W.; Packard, S.D.; Hsu, D.W.; Hochberg, F.H.; Fischman, A.J.; Rosen, B.R. High microvascular blood volume is associated with high glucose uptake and tumor angiogenesis in human gliomas. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2000, 6, 2189–2200. [Google Scholar]
- Mirus, M.; Tokalov, S.V.; Abramyuk, A.; Heinold, J.; Prochnow, V.; Zophel, K.; Kotzerke, J.; Abolmaali, N. Noninvasive assessment and quantification of tumor vascularization using [18F]FDG-PET/CT and CE-CT in a tumor model with modifiable angiogenesis-an animal experimental prospective cohort study. EJNMMI Res. 2019, 9, 55. [Google Scholar] [CrossRef] [PubMed]
- Toledo, M.; Busquets, S.; Sirisi, S.; Serpe, R.; Orpi, M.; Coutinho, J.; Martinez, R.; Lopez-Soriano, F.J.; Argiles, J.M. Cancer cachexia: Physical activity and muscle force in tumour-bearing rats. Oncol. Rep. 2011, 25, 189–193. [Google Scholar] [PubMed]
- Stigliano, R.V.; Shubitidze, F.; Petryk, J.D.; Shoshiashvili, L.; Petryk, A.A.; Hoopes, P.J. Mitigation of eddy current heating during magnetic nanoparticle hyperthermia therapy. Int. J. Hyperth. 2016, 32, 735–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yameen, B.; Choi, W.I.; Vilos, C.; Swami, A.; Shi, J.; Farokhzad, O.C. Insight into nanoparticle cellular uptake and intracellular targeting. J. Control. Release 2014, 190, 485–499. [Google Scholar] [CrossRef] [Green Version]
- Belanova, A.A.; Gavalas, N.; Makarenko, Y.M.; Belousova, M.M.; Soldatov, A.V.; Zolotukhin, P.V. Physicochemical Properties of Magnetic Nanoparticles: Implications for Biomedical Applications In Vitro and In Vivo. Oncol. Res. Treat. 2018, 41, 139–143. [Google Scholar] [CrossRef]
- Rego, G.N.A.; Mamani, J.B.; Souza, T.K.F.; Nucci, M.P.; Silva, H.R.D.; Gamarra, L.F. Therapeutic evaluation of magnetic hyperthermia using Fe3O4-aminosilane-coated iron oxide nanoparticles in glioblastoma animal model. Einstein (Sao Paulo) 2019, 17, eAO4786. [Google Scholar] [CrossRef] [Green Version]
- Youhannayee, M.; Nakhaei-Rad, S.; Haghighi, F.; Klauke, K.; Janiak, C.; Ahmadian, M.R.; Rabenalt, R.; Albers, P.; Getzlaff, M. Physical characterization and uptake of iron oxide nanoparticles of different prostate cancer cells. J. Magn. Magn. Mater. 2019, 473, 205–214. [Google Scholar] [CrossRef]
- Shubitidze, F.; Kekalo, K.; Stigliano, R.; Baker, I. Magnetic nanoparticles with high specific absorption rate of electromagnetic energy at low field strength for hyperthermia therapy. J. Appl. Phys. 2015, 117, 094302. [Google Scholar] [CrossRef]
- Kekalo, K.; Baker, I.; Meyers, R.; Shyong, J. Magnetic Nanoparticles with High Specific Absorption Rate at Low Alternating Magnetic Field. Nano Life 2015, 5. [Google Scholar] [CrossRef]
- Yuan, Y.; Tasciuc, D.-A.B. Comparison between experimental and predicted specific absorption rate of functionalized iron oxide nanoparticle suspensions. J. Magn. Magn. Mater. 2011, 323, 2463–2469. [Google Scholar] [CrossRef]
- Xu, H.; Pan, Y. Experimental Evaluation on the Heating Efficiency of Magnetoferritin Nanoparticles in an Alternating Magnetic Field. Nanomaterials 2019, 9, 1457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ludwig, R.; Stapf, M.; Dutz, S.; Muller, R.; Teichgraber, U.; Hilger, I. Structural properties of magnetic nanoparticles determine their heating behavior—An estimation of the in vivo heating potential. Nanoscale Res. Lett. 2014, 9, 602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murayama, S.; Nishiyama, T.; Takagi, K.; Ishizuka, F.; Santa, T.; Kato, M. Delivery, stabilization, and spatiotemporal activation of cargo molecules in cells with positively charged nanoparticles. Chem. Commun. 2012, 48, 11461–11463. [Google Scholar] [CrossRef]
- Li, L.; Jiang, W.; Luo, K.; Song, H.; Lan, F.; Wu, Y.; Gu, Z. Superparamagnetic iron oxide nanoparticles as MRI contrast agents for non-invasive stem cell labeling and tracking. Theranostics 2013, 3, 595–615. [Google Scholar] [CrossRef]
- Hu, L.; Mao, Z.; Gao, C. Colloidal particles for cellular uptake and delivery. J. Mater. Chem. 2009, 19, 3108–3115. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, M.; Portney, N.G.; Cui, D.; Budak, G.; Ozbay, E.; Ozkan, M.; Ozkan, C.S. Zeta potential: A surface electrical characteristic to probe the interaction of nanoparticles with normal and cancer human breast epithelial cells. Biomed. Microdevices 2008, 10, 321–328. [Google Scholar] [CrossRef]
- Bowman, C.L.; Lohr, J.W. Mechanotransducing ion channels in C6 glioma cells. Glia 1996, 18, 161–176. [Google Scholar] [CrossRef]
- Rivet, C.J.; Yuan, Y.; Gilbert, R.J.; Borca-Tasciuc, D.A. Effect of magnetic nanoparticle heating on cortical neuron viability. Int. J. Hyperth. 2014, 30, 79–85. [Google Scholar] [CrossRef]
- Zhong, L.; Chen, L.; Lv, S.; Li, Q.; Chen, G.; Luo, W.; Zhou, P.; Li, G. Efficacy of moderately hypofractionated simultaneous integrated boost intensity-modulated radiotherapy combined with temozolomide for the postoperative treatment of glioblastoma multiforme: A single-institution experience. Radiat. Oncol. 2019, 14, 104. [Google Scholar] [CrossRef]
- Sulman, E.P.; Ismaila, N.; Armstrong, T.S.; Tsien, C.; Batchelor, T.T.; Cloughesy, T.; Galanis, E.; Gilbert, M.; Gondi, V.; Lovely, M.; et al. Radiation Therapy for Glioblastoma: American Society of Clinical Oncology Clinical Practice Guideline Endorsement of the American Society for Radiation Oncology Guideline. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2017, 35, 361–369. [Google Scholar] [CrossRef] [Green Version]
- Grauer, O.; Jaber, M.; Hess, K.; Weckesser, M.; Schwindt, W.; Maring, S.; Wölfer, J.; Stummer, W. Combined intracavitary thermotherapy with iron oxide nanoparticles and radiotherapy as local treatment modality in recurrent glioblastoma patients. J. Neuro Oncol. 2019, 141, 83–94. [Google Scholar] [CrossRef] [Green Version]
- Jordan, A.; Scholz, R.; Maier-Hauff, K.; van Landeghem, F.K.H.; Waldoefner, N.; Teichgraeber, U.; Pinkernelle, J.; Bruhn, H.; Neumann, F.; Thiesen, B.; et al. The effect of thermotherapy using magnetic nanoparticles on rat malignant glioma. J. Neuro Oncol. 2006, 78, 7–14. [Google Scholar] [CrossRef]
- James, M.L.; Gambhir, S.S. A molecular imaging primer: Modalities, imaging agents, and applications. Physiol. Rev. 2012, 92, 897–965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frosina, G. Positron emission tomography of high-grade gliomas. J. Neurooncol. 2016, 127, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Fischer, B.M.; Olsen, M.W.; Ley, C.D.; Klausen, T.L.; Mortensen, J.; Hojgaard, L.; Kristjansen, P.E. How few cancer cells can be detected by positron emission tomography? A frequent question addressed by an in vitro study. Eur. J. Nucl. Med. Mol. Imaging 2006, 33, 697–702. [Google Scholar] [CrossRef]
- Warnock, G.; Turtoi, A.; Blomme, A.; Bretin, F.; Bahri, M.A.; Lemaire, C.; Libert, L.C.; Seret, A.E.; Luxen, A.; Castronovo, V.; et al. In vivo PET/CT in a human glioblastoma chicken chorioallantoic membrane model: A new tool for oncology and radiotracer development. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2013, 54, 1782–1788. [Google Scholar] [CrossRef] [Green Version]
- Alf, M.F.; Wyss, M.T.; Buck, A.; Weber, B.; Schibli, R.; Kramer, S.D. Quantification of brain glucose metabolism by 18F-FDG PET with real-time arterial and image-derived input function in mice. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2013, 54, 132–138. [Google Scholar] [CrossRef] [Green Version]
- Colavolpe, C.; Chinot, O.; Metellus, P.; Mancini, J.; Barrie, M.; Bequet-Boucard, C.; Tabouret, E.; Mundler, O.; Figarella-Branger, D.; Guedj, E. FDG-PET predicts survival in recurrent high-grade gliomas treated with bevacizumab and irinotecan. Neuro Oncol. 2012, 14, 649–657. [Google Scholar] [CrossRef] [Green Version]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates; Acad. Press: Cambridge, MA, USA, 1986. [Google Scholar]










| Magnetic Field (Gauss) | Frequeny (kHz) | Mean Time to Achieve of 43°C (s) | N |
|---|---|---|---|
| 300 | 557 | 14 ± 2 | 5 |
| 300 | 420 | 15 ± 3 | 5 |
| 300 | 364 | 23 ± 4 | 5 |
| 300 | 309 | 26 ± 4 | 5 |
| 200 | 874 | 32 ± 5 | 5 |
| 200 | 557 | 57 ± 4 | 5 |
| 200 | 420 | 58 ± 4 | 5 |
| 200 | 364 | 104 ± 5 | 5 |
| 200 | 309 | 119 ± 7 | 5 |
| Magnetic Field (Gauss) | Frequency (kHz) | SAR Mean (W/g) | SD (W/g) | N |
|---|---|---|---|---|
| 100 | 309 | 3.789 | 0.137 | 5 |
| 364 | 6.785 | 0.736 | 5 | |
| 420 | 8.295 | 0.920 | 5 | |
| 557 | 11.078 | 0.403 | 5 | |
| 874 | 17.045 | 1.306 | 5 | |
| 200 | 309 | 54.757 | 1.460 | 5 |
| 364 | 61.796 | 3.039 | 5 | |
| 420 | 79.117 | 1.101 | 5 | |
| 557 | 82.772 | 3.548 | 5 | |
| 874 | 175.112 | 10.897 | 5 | |
| 300 | 309 | 169.297 | 5.097 | 5 |
| 364 | 185.464 | 7.646 | 5 | |
| 420 | 238.775 | 0.350 | 5 | |
| 557 | 320.070 | 22.818 | 5 |
| MHT Applications | Groups | Mean (photons/s) | SD (photons/s) | n |
|---|---|---|---|---|
| One | C6 | 4.070 × 109 | 2.643 × 108 | 4 |
| C6 with SPIONa | 4.030 × 109 | 1.479 × 108 | 4 | |
| C6-f1-B | 4.365 × 109 | 2.763 × 108 | 4 | |
| C6-f1-B with SPIONa | 1.748 × 109 | 1.124 × 108 | 4 | |
| C6-f2-B | 4.375 × 109 | 2.763 × 108 | 4 | |
| C6-f2-B with SPIONa | 8.730 × 108 | 5.501 × 107 | 4 | |
| Two | C6 | 5.242 × 109 | 3.301 × 108 | 4 |
| C6-SPIONa | 5.237 × 109 | 3.297 × 108 | 4 | |
| C6-f1-B | 5.237 × 109 | 3.297 × 108 | 4 | |
| C6-f1-B with SPIONa | 1.309 × 109 | 8.243 × 107 | 4 | |
| C6-f2-B | 5.237 × 109 | 3.297 × 108 | 4 | |
| C6-f2-B with SPIONa | 6.546 × 108 | 4.121 × 107 | 4 | |
| Three | C6 | 6.022 × 109 | 3.792 × 108 | 4 |
| C6-SPIONa | 6.110 × 109 | 3.847 × 108 | 4 | |
| C6-f1-B | 6.241 × 109 | 3.929 × 108 | 4 | |
| C6-f1-B with SPIONa | 4.364 × 107 | 2.748 × 106 | 4 | |
| C6-f2-B | 6.197 × 109 | 3.902 × 108 | 4 | |
| C6-f2-B with SPIONa | 5.673 × 108 | 3.57 × 107 | 4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rego, G.N.A.; Nucci, M.P.; Mamani, J.B.; Oliveira, F.A.; Marti, L.C.; Filgueiras, I.S.; Ferreira, J.M.; Real, C.C.; Faria, D.d.P.; Espinha, P.L.; et al. Therapeutic Efficiency of Multiple Applications of Magnetic Hyperthermia Technique in Glioblastoma Using Aminosilane Coated Iron Oxide Nanoparticles: In Vitro and In Vivo Study. Int. J. Mol. Sci. 2020, 21, 958. https://doi.org/10.3390/ijms21030958
Rego GNA, Nucci MP, Mamani JB, Oliveira FA, Marti LC, Filgueiras IS, Ferreira JM, Real CC, Faria DdP, Espinha PL, et al. Therapeutic Efficiency of Multiple Applications of Magnetic Hyperthermia Technique in Glioblastoma Using Aminosilane Coated Iron Oxide Nanoparticles: In Vitro and In Vivo Study. International Journal of Molecular Sciences. 2020; 21(3):958. https://doi.org/10.3390/ijms21030958
Chicago/Turabian StyleRego, Gabriel N. A., Mariana P. Nucci, Javier B. Mamani, Fernando A. Oliveira, Luciana C. Marti, Igor S. Filgueiras, João M. Ferreira, Caroline C. Real, Daniele de Paula Faria, Paloma L. Espinha, and et al. 2020. "Therapeutic Efficiency of Multiple Applications of Magnetic Hyperthermia Technique in Glioblastoma Using Aminosilane Coated Iron Oxide Nanoparticles: In Vitro and In Vivo Study" International Journal of Molecular Sciences 21, no. 3: 958. https://doi.org/10.3390/ijms21030958
APA StyleRego, G. N. A., Nucci, M. P., Mamani, J. B., Oliveira, F. A., Marti, L. C., Filgueiras, I. S., Ferreira, J. M., Real, C. C., Faria, D. d. P., Espinha, P. L., Fantacini, D. M. C., Souza, L. E. B., Covas, D. T., Buchpiguel, C. A., & Gamarra, L. F. (2020). Therapeutic Efficiency of Multiple Applications of Magnetic Hyperthermia Technique in Glioblastoma Using Aminosilane Coated Iron Oxide Nanoparticles: In Vitro and In Vivo Study. International Journal of Molecular Sciences, 21(3), 958. https://doi.org/10.3390/ijms21030958