The Intravitreal Injection of Lanosterol Nanoparticles Rescues Lens Structure Collapse at an Early Stage in Shumiya Cataract Rats
Abstract
:1. Introduction
2. Results
2.1. Design of Dispersions Containing Lanosterol Nanoparticles
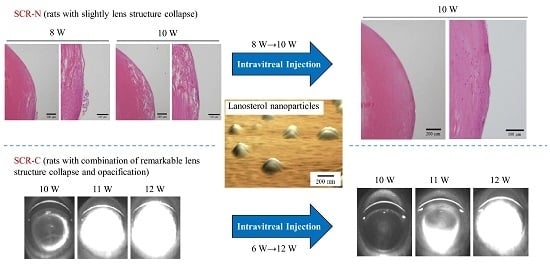
2.2. Safety Evaluation of Intravitreal Injections of LAN-NPs
2.3. Changes in Lanosterol Levels, Opacity, and Structure in the Lenses of SCR-N and SCR-C with Aging
2.4. Therapeutic Potential of Lanosterol in SCR-N and SCR-C Injected Intravitreally with LAN-NPs
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Chemicals
4.3. Preparation of LAN-NPs
4.4. Measurement of Lanosterol by the LC-CAD Method
4.5. Evaluation of the Characteristics of the LAN-NPs
4.6. Measurement of Lanosterol content in Rat Lenses
4.7. Measurement of Toxicity using Cultured Human Lens Cells
4.8. Scheimpflug Slit Images in the SCR
4.9. Hematoxylin and Eosin (H&E) Staining of the Lens
4.10. Measurement of Cararact-Related Factors
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AFM | atomic force microscope |
| DDS | drug delivery systems |
| (H&E) | hematoxylin and eosin |
| HLE | human lens epithelial cell line SRA 01/04 |
| HPβCD | 2-hydroxypropyl-β-cyclodextrin |
| IFN-γ | interferon-γ |
| LAN-NPs | intravitreal injection formulation containing lanosterol nanoparticles |
| LPO | lipid peroxidation |
| LPS | lipopolysaccharide |
| MC | methylcellulose |
| NO | nitric oxide |
| PLGA | Poly(lactic-co-glycolic acid) |
| SCR-C | cataractous Shumiya cataract rat |
| SCR-N | non-cataract Shumiya cataract rat |
| S.D. | standard deviation |
| S.E. | standard error |
References
- Klein, B.E.; Klein, R.; Lee, K.E. Incidence of age-related cataract over a 10-year interval: The Beaver Dam Eye Study. Ophthalmology 2002, 109, 2052–2057. [Google Scholar] [CrossRef]
- Congdon, N.; Vingerling, J.R.; Klein, B.E.; West, S.; Friedman, D.S.; Kempen, J.; O’Colmain, B.; Wu, S.Y.; Taylor, H.R.; Eye Diseases Prevalence Research Group. Prevalence of cataract and pseudophakia/aphakia among adults in the United States. Arch. Ophthalmol. 2004, 122, 487–494. [Google Scholar] [PubMed]
- Zhao, L.; Chen, X.J.; Zhu, J.; Xi, Y.B.; Yang, X.; Hu, L.D.; Ouyang, H.; Patel, S.H.; Jin, X.; Lin, D.; et al. Lanosterol reverses protein aggregation in cataracts. Nature 2015, 523, 607–611. [Google Scholar] [CrossRef] [PubMed]
- Daszynski, D.M.; Santhoshkumar, P.; Phadte, A.S.; Sharma, K.K.; Zhong, H.A.; Lou, M.F.; Kador, P.F. Failure of Oxysterols Such as Lanosterol to Restore Lens Clarity from Cataracts. Sci. Rep. 2019, 9, 8459. [Google Scholar] [CrossRef]
- Shanmugam, P.M.; Barigali, A.; Kadaskar, J.; Borgohain, S.; Mishra, D.K.; Ramanjulu, R.; Minija, C.K. Effect of lanosterol on human cataract nucleus. Indian J. Ophthalmol. 2015, 63, 888–890. [Google Scholar] [CrossRef]
- Kang, H.; Yang, Z.; Zhou, R. Lanosterol disrupts aggregation of human gammaD-crystallin by binding to the hydrophobic dimerization interface. J. Am. Chem. Soc. 2018, 140, 8479–8486. [Google Scholar] [CrossRef]
- Hua, H.; Yang, T.; Huang, L.; Chen, R.; Li, M.; Zou, Z.; Wang, N.; Yang, D.; Liu, Y. Protective Effects of Lanosterol Synthase Up-Regulation in UV-B-Induced Oxidative Stress. Front Pharmacol. 2019, 10, 947. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Li, C.; Muhemaitia, P. Impediment of selenite-induced cataract in rats by combinatorial drug laden liposomal preparation. Libyan J. Med. 2019, 14, 1548252. [Google Scholar] [CrossRef] [Green Version]
- Cholkar, K.; Patel, A.; Vadlapudi, A.D.; Mitra, A.K. Novel Nanomicellar formulation approaches for anterior and posterior segment ocular drug delivery. Recent Pat. Nanomed. 2012, 2, 82–95. [Google Scholar] [CrossRef]
- Patel, A.; Cholkar, K.; Agrahari, V.; Mitra, A.K. Ocular drug delivery systems: An overview. World J. Pharmacol. 2013, 2, 47–64. [Google Scholar] [CrossRef]
- Fangueiro, J.F.; Veiga, F.; Silva, A.M.; Souto, E.B. Ocular Drug Delivery–New Strategies for Targeting Anterior and Posterior Segments of the Eye. Curr. Pharm. Des. 2016, 22, 1135–1146. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Ogata, F.; Otake, H.; Nakazawa, Y.; Kawasaki, N. Energy-dependent endocytosis is responsible for drug transcorneal penetration following the instillation of ophthalmic formulations containing indomethacin nanoparticles. Int. J. Nanomed. 2019, 14, 1213–1227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagai, N.; Ogata, F.; Otake, H.; Nakazawa, Y.; Kawasaki, N. Design of a transdermal formulation containing raloxifene nanoparticles for osteoporosis treatment. Int. J. Nanomed. 2018, 13, 5215–5229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagai, N.; Yoshioka, C.; Ito, Y.; Funakami, Y.; Nishikawa, H.; Kawabata, A. Intravenous Administration of Cilostazol Nanoparticles Ameliorates Acute Ischemic Stroke in a Cerebral Ischemia/Reperfusion-Induced Injury Model. Int. J. Mol. Sci. 2015, 16, 29329–29344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagai, N.; Ono, H.; Hashino, M.; Ito, Y.; Okamoto, N.; Shimomura, Y. Improved corneal toxicity and permeability of tranilast by the preparation of ophthalmic formulations containing its nanoparticles. J. Oleo Sci. 2014, 63, 177–186. [Google Scholar] [CrossRef] [Green Version]
- Nagai, N.; Ito, Y. Effect of Solid Nanoparticle of Indomethacin on Therapy for Rheumatoid Arthritis in Adjuvant-Induced Arthritis Rat. Biol. Pharm. Bull. 2014, 37, 1109–1118. [Google Scholar] [CrossRef] [Green Version]
- Shumiya, S. Establishment of the hereditary cataract rat strain (SCR) and genetic analysis. Lab. Anim. Sci. 1995, 45, 671–673. [Google Scholar]
- Nagai, N.; Ito, Y.; Takeuchi, N.; Usui, S.; Hirano, K. Comparison of the Mechanisms of Cataract Development Involving Differences in Ca(2+) Regulation in Lenses among Three Hereditary Cataract Model Rats. Biol. Pharm. Bull. 2008, 31, 1990–1995. [Google Scholar] [CrossRef] [Green Version]
- Mori, K.; Yoshioka, N.; Kondo, Y.; Takeuchi, T.; Yamashita, H. Catalyticallyactive, magnetically separable, and water-soluble FePt nanoparticles modi-fied with cyclodextrin for aqueous hydrogenation reactions. Green Chem. 2009, 11, 1337–1342. [Google Scholar] [CrossRef]
- Nagai, N.; Ito, Y.; Okamoto, N.; Shimomura, Y. A nanoparticle formulation reduces the corneal toxicity of indomethacin eye drops and enhances its corneal permeability. Toxicology 2014, 319, 53–62. [Google Scholar] [CrossRef]
- Wells, M.R.; Kraus, K.; Batter, D.K.; Blunt, D.G.; Weremowitz, J.; Lynch, S.E.; Antoni-ades, H.N.; Hansson, H.A. Gel matrix vehicles for growth factor applicationin nerve gap injuries repaired with tubes: A comparison of biomatrix, collagen, and methylcellulose. Exp. Neurol. 1997, 146, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Tate, M.C.; Shear, D.A.; Hoffman, S.W.; Stein, D.G.; LaPlaca, M.C. Bio-compatibility of methylcellulose-based constructs designed for intracerebralgelation following experimental traumatic brain injury. Biomaterials 2001, 22, 1113–1123. [Google Scholar] [CrossRef]
- Gupta, D.; Tator, C.H.; Shoichet, M.S. Fast-gelling injectable blend of hyaluro-nan and methylcellulose for intrathecal, localized delivery to the injured spinalcord. Biomaterials 2006, 27, 2370–2379. [Google Scholar] [CrossRef] [PubMed]
- Jansen, T.; Xhonneux, B.; Mesens, J.; Borgers, M. Beta-cyclodextrins as vehi-cles in eye-drop formulations: An evaluation of their effects on rabbit cornealepithelium. Lens Eye Toxic. Res. 1990, 7, 459–468. [Google Scholar]
- Mori, M.; Li, G.; Abe, I.; Nakayama, J.; Guo, Z.; Sawashita, J.; Ugawa, T.; Nishizono, S.; Serikawa, T.; Higuchi, K.; et al. Lanosterol synthase mutations cause cholesterol deficiency-associated cataracts in the Shumiya cataract rat. J. Clin. Investig. 2006, 116, 395–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okano, T.; Uga, S.; Ishikawa, S.; Shumiya, S. Histopathological study of hereditary cataractous lenses in SCR strain rat. Exp. Eye Res. 1993, 57, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Hightower, K.R.; McCready, J.P. Effect of selenite on epithelium of cultured rabbit lens. Investig. Ophthalmol. Vis. Sci. 1991, 32, 406–409. [Google Scholar]
- Okano, T.; Uga, S.; Ishikawa, S.; Hara, A.; Shumiya, S. Lens reconstruction after cataract in SCR rat. Jpn. J. Opthalmol. 1999, 43, 363–367. [Google Scholar] [CrossRef]
- Nagai, N.; Ito, Y.; Shibata, T.; Kubo, E.; Sasaki, H. A positive feedback loop between nitric oxide and amyloid β (1-42) accelerates mitochondrial damage in human lens epithelial cells. Toxicology 2017, 381, 19–30. [Google Scholar] [CrossRef]
- Inomata, M.; Hayashi, M.; Shumiya, S.; Kawashima, S.; Ito, Y. Involvement of inducible nitric oxide synthase in cataract formation in Shumiya cataract rat (SCR). Curr. Eye Res. 2001, 23, 307–311. [Google Scholar] [CrossRef]
- Inomata, M.; Hayashi, M.; Shumiya, S.; Kawashima, S.; Ito, Y. Aminoguanidine-treatment results in the inhibition of lens opacification and calpain-mediated proteolysis in Shumiya cataract rats (SCR). J. Biochem. 2000, 128, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Liu, Y.; Fukuhata, T.; Ito, Y. Inhibitors of Inducible Nitric Oxide Synthase Prevent Damage to Human Lens Epithelial Cells Induced by Interferon-Gamma and Lipopolysaccharide. Biol. Pharm. Bull. 2006, 29, 2077–2081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishii, M.; Fukuoka, Y.; Deguchi, S.; Otake, H.; Tanino, T.; Nagai, N. Energy-Dependent Endocytosis is Involved in the Absorption of Indomethacin Nanoparticles in the Small Intestine. Int. J. Mol. Sci. 2019, 20, 476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagai, N.; Ogata, F.; Ishii, M.; Fukuoka, Y.; Otake, H.; Nakazawa, Y.; Kawasaki, N. Involvement of Endocytosis in the Transdermal Penetration Mechanism of Ketoprofen Nanoparticles. Int. J. Mol. Sci. 2018, 19, 2138. [Google Scholar] [CrossRef] [Green Version]
- Nagai, N.; Kotani, S.; Mano, Y.; Ueno, A.; Ito, Y.; Kitaba, T.; Takata, T.; Fujii, N. Ferulic Acid Suppresses Amyloid β Production in the Human Lens Epithelial Cell Stimulated with Hydrogen Peroxide. BioMed Res. Int. 2017, 2017, 1–9. [Google Scholar] [CrossRef]
- Nagai, N.; Ito, Y.; Takeuchi, N. Effect of disulfiram eye drops on lipid peroxide formation via excessive nitric oxide in lenses of hereditary cataract ICR/f rats. Biol. Pharm. Bull. 2008, 31, 981–985. [Google Scholar] [CrossRef] [Green Version]
- Deguchi, S.; Otake, H.; Nakazawa, Y.; Hiramatsu, N.; Yamamoto, N.; Nagai, N. Ophthalmic Formulation Containing Nilvadipine Nanoparticles Prevents Retinal Dysfunction in Rats Injected with Streptozotocin. Int. J. Mol. Sci. 2017, 18, 2720. [Google Scholar] [CrossRef] [Green Version]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagai, N.; Fukuoka, Y.; Sato, K.; Otake, H.; Taga, A.; Oka, M.; Hiramatsu, N.; Yamamoto, N. The Intravitreal Injection of Lanosterol Nanoparticles Rescues Lens Structure Collapse at an Early Stage in Shumiya Cataract Rats. Int. J. Mol. Sci. 2020, 21, 1048. https://doi.org/10.3390/ijms21031048
Nagai N, Fukuoka Y, Sato K, Otake H, Taga A, Oka M, Hiramatsu N, Yamamoto N. The Intravitreal Injection of Lanosterol Nanoparticles Rescues Lens Structure Collapse at an Early Stage in Shumiya Cataract Rats. International Journal of Molecular Sciences. 2020; 21(3):1048. https://doi.org/10.3390/ijms21031048
Chicago/Turabian StyleNagai, Noriaki, Yuya Fukuoka, Kanta Sato, Hiroko Otake, Atsushi Taga, Mikako Oka, Noriko Hiramatsu, and Naoki Yamamoto. 2020. "The Intravitreal Injection of Lanosterol Nanoparticles Rescues Lens Structure Collapse at an Early Stage in Shumiya Cataract Rats" International Journal of Molecular Sciences 21, no. 3: 1048. https://doi.org/10.3390/ijms21031048
APA StyleNagai, N., Fukuoka, Y., Sato, K., Otake, H., Taga, A., Oka, M., Hiramatsu, N., & Yamamoto, N. (2020). The Intravitreal Injection of Lanosterol Nanoparticles Rescues Lens Structure Collapse at an Early Stage in Shumiya Cataract Rats. International Journal of Molecular Sciences, 21(3), 1048. https://doi.org/10.3390/ijms21031048