Surface Functionalization with Polyethylene Glycol and Polyethyleneimine Improves the Performance of Graphene-Based Materials for Safe and Efficient Intracellular Delivery by Laser-Induced Photoporation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physicochemical Characterization of GQD and rGO Before and after Surface Functionalization
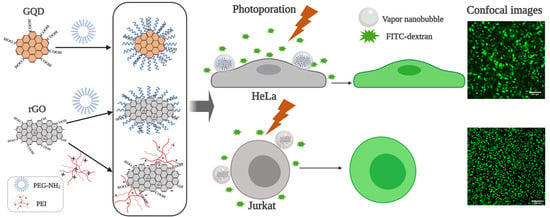
2.2. Photoporation of Adherent Cells with Polymer-Modified Graphene-Based Nanoparticles
2.3. Photoporation of Suspension Cells with Polymer-Modified Graphene-Based Nanoparticles
2.4. Exploring Intracellular Delivery of Different Sizes of Cargos
2.5. Intracellular Delivery by NIR Laser Induced-Photoporation with rGO-PEI
3. Materials and Methods
3.1. Cell Culture
3.2. GQD and GQD-PEG Synthesis Method
3.3. rGO, rGO-PEG and rGO-PEI Synthesis Method
3.4. Ultraviolet (UV)-NIR Spectrophotometry, DLS and NanoSight Measurements
3.5. SEM Imaging
3.6. Raman Measurement
3.7. Laser-Induced Photoporation
3.8. VNB Generation and Visualization in Living Cells
3.9. Confocal Microscopy Imaging
3.10. Flow Cytometry
3.11. Cell Viability Assay by CellTiter Glo®
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stewart, M.P.; Langer, R.; Jensen, K.F. Intracellular delivery by membrane disruption: Mechanisms, strategies, and concepts. Chem. Rev. 2018, 118, 7409–7531. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.P.; Sharei, A.; Ding, X.; Sahay, G.; Langer, R.; Jensen, K.F. In vitro and ex vivo strategies for intracellular delivery. Nature 2016, 538, 183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fajrial, A.K.; Ding, X. Advanced nanostructures for cell membrane poration. Nanotechnology 2019, 30, 264002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varela-Rohena, A.; Carpenito, C.; Perez, E.E.; Richardson, M.; Parry, R.V.; Milone, M.; Scholler, J.; Hao, X.; Mexas, A.; Carroll, R.G. Genetic engineering of T cells for adoptive immunotherapy. Immunol. Res. 2008, 42, 166–181. [Google Scholar] [CrossRef] [Green Version]
- Wayteck, L.; Xiong, R.; Braeckmans, K.; De Smedt, S.C.; Raemdonck, K. Comparing photoporation and nucleofection for delivery of small interfering RNA to cytotoxic T cells. J. Control. Release 2017, 267, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Rogers, W.J.; Meyer, C.H.; Kramer, C.M. Technology insight: In vivo cell tracking by use of MRI. Nat. Rev. Cardiol. 2006, 3, 554. [Google Scholar] [CrossRef]
- Tachibana, Y.; Enmi, J.; Mahara, A.; Iida, H.; Yamaoka, T. Design and characterization of a polymeric MRI contrast agent based on PVA for in vivo living-cell tracking. Contrast Media Mol. Imaging 2010, 5, 309–317. [Google Scholar] [CrossRef]
- Gilleron, J.; Querbes, W.; Zeigerer, A.; Borodovsky, A.; Marsico, G.; Schubert, U.; Manygoats, K.; Seifert, S.; Andree, C.; Stöter, M. Image-based analysis of lipid nanoparticle–mediated siRNA delivery, intracellular trafficking and endosomal escape. Nat. Biotechnol. 2013, 31, 638. [Google Scholar] [CrossRef]
- Vermeulen, L.M.; Brans, T.; Samal, S.K.; Dubruel, P.; Demeester, J.; De Smedt, S.C.; Remaut, K.; Braeckmans, K. Endosomal size and membrane leakiness influence proton sponge-based rupture of endosomal vesicles. ACS Nano 2018, 12, 2332–2345. [Google Scholar] [CrossRef] [Green Version]
- Lönn, P.; Dowdy, S.F. Cationic PTD/CPP-mediated macromolecular delivery: Charging into the cell. Expert Opin. Drug Deliv. 2015, 12, 1627–1636. [Google Scholar] [CrossRef]
- Bechara, C.; Sagan, S. Cell-penetrating peptides: 20 years later, where do we stand? FEBS Lett. 2013, 587, 1693–1702. [Google Scholar] [CrossRef] [PubMed]
- Li, S. Electroporation gene therapy: New developments in vivo and in vitro. Curr. Gene Ther. 2004, 4, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Stewart, M.P.; Sharei, A.; Weaver, J.C.; Langer, R.S.; Jensen, K.F. High-throughput nuclear delivery and rapid expression of DNA via mechanical and electrical cell-membrane disruption. Nat. Biomed. Eng. 2017, 1, 0039. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Kizer, M.; Rada, M.; Sage, J.; Wang, X.; Cheon, D.-J.; Chung, A.J. Intracellular delivery of nanomaterials via an inertial microfluidic cell hydroporator. Nano Lett. 2018, 18, 2705–2710. [Google Scholar] [CrossRef] [PubMed]
- Xiong, R.; Raemdonck, K.; Peynshaert, K.; Lentacker, I.; De Cock, I.; Demeester, J.; De Smedt, S.C.; Skirtach, A.G.; Braeckmans, K. Comparison of gold nanoparticle mediated photoporation: Vapor nanobubbles outperform direct heating for delivering macromolecules in live cells. ACS Nano 2014, 8, 6288–6296. [Google Scholar] [CrossRef]
- Xiong, R.; Samal, S.K.; Demeester, J.; Skirtach, A.G.; De Smedt, S.C.; Braeckmans, K. Laser-assisted photoporation: Fundamentals, technological advances and applications. Adv. Phys. X 2016, 1, 596–620. [Google Scholar] [CrossRef] [Green Version]
- Lapotko, D. Optical excitation and detection of vapor bubbles around plasmonic nanoparticles. Opt. Express 2009, 17, 2538–2556. [Google Scholar] [CrossRef]
- Umebayashi, Y.; Miyamoto, Y.; Wakita, M.; Kobayashi, A.; Nishisaka, T. Elevation of plasma membrane permeability on laser irradiation of extracellular latex particles. J. Biochem. 2003, 134, 219–224. [Google Scholar] [CrossRef]
- Xiong, R.; Joris, F.; Liang, S.; De Rycke, R.; Lippens, S.; Demeester, J.; Skirtach, A.; Raemdonck, K.; Himmelreich, U.; De Smedt, S.C. Cytosolic Delivery of Nanolabels Prevents Their Asymmetric Inheritance and Enables Extended Quantitative in Vivo Cell Imaging. Nano Lett. 2016, 16, 5975–5986. [Google Scholar] [CrossRef] [Green Version]
- Dreaden, E.C.; Alkilany, A.M.; Huang, X.; Murphy, C.J.; El-Sayed, M.A. The golden age: Gold nanoparticles for biomedicine. Chem. Soc. Rev. 2012, 41, 2740–2779. [Google Scholar] [CrossRef] [Green Version]
- Pan, Y.; Neuss, S.; Leifert, A.; Fischler, M.; Wen, F.; Simon, U.; Schmid, G.; Brandau, W.; Jahnen-Dechent, W. Size-dependent cytotoxicity of gold nanoparticles. Small 2007, 3, 1941–1949. [Google Scholar] [CrossRef] [PubMed]
- Schaeublin, N.M.; Braydich-Stolle, L.K.; Schrand, A.M.; Miller, J.M.; Hutchison, J.; Schlager, J.J.; Hussain, S.M. Surface charge of gold nanoparticles mediates mechanism of toxicity. Nanoscale 2011, 3, 410–420. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; El-Sayed, M.A. Gold nanoparticles: Optical properties and implementations in cancer diagnosis and photothermal therapy. J. Adv. Res. 2010, 1, 13–28. [Google Scholar] [CrossRef] [Green Version]
- Yang, K.; Zhang, S.; Zhang, G.; Sun, X.; Lee, S.-T.; Liu, Z. Graphene in mice: Ultrahigh in vivo tumor uptake and efficient photothermal therapy. Nano Lett. 2010, 10, 3318–3323. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-W.; Su, Y.-L.; Hu, S.-H.; Chen, S.-Y. Functionalized graphene nanocomposites for enhancing photothermal therapy in tumor treatment. Adv. Drug Deliv. Rev. 2016, 105, 190–204. [Google Scholar] [CrossRef]
- Yu, X.; Cheng, H.; Zhang, M.; Zhao, Y.; Qu, L.; Shi, G. Graphene-based smart materials. Nat. Rev. Mater. 2017, 2, 17046. [Google Scholar] [CrossRef]
- Liu, J.; Xiong, R.; Brans, T.; Lippens, S.; Parthoens, E.; Zanacchi, F.C.; Magrassi, R.; Singh, S.K.; Kurungot, S.; Szunerits, S. Repeated photoporation with graphene quantum dots enables homogeneous labeling of live cells with extrinsic markers for fluorescence microscopy. Light Sci. Appl. 2018, 7, 47. [Google Scholar] [CrossRef] [Green Version]
- Pansare, V.J.; Hejazi, S.; Faenza, W.J.; Prud’homme, R.K. Review of long-wavelength optical and NIR imaging materials: Contrast agents, fluorophores, and multifunctional nano carriers. Chem. Mater. 2012, 24, 812–827. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Zhang, F. NIR luminescent nanomaterials for biomedical imaging. J. Mater. Chem. B 2014, 2, 2422–2443. [Google Scholar] [CrossRef]
- Yue, Y.; Jin, F.; Deng, R.; Cai, J.; Dai, Z.; Lin, M.C.; Kung, H.-F.; Mattebjerg, M.A.; Andresen, T.L.; Wu, C. Revisit complexation between DNA and polyethylenimine—Effect of length of free polycationic chains on gene transfection. J. Control. Release 2011, 152, 143–151. [Google Scholar] [CrossRef]
- Zaharie-Butucel, D.; Potara, M.; Craciun, A.; Boukherroub, R.; Szunerits, S.; Astilean, S. Revealing the structure and functionality of graphene oxide and reduced graphene oxide/pyrene carboxylic acid interfaces by correlative spectral and imaging analysis. Phys. Chem. Chem. Phys. 2017, 19, 16038–16046. [Google Scholar] [CrossRef] [PubMed]
- Lukianova-Hleb, E.Y.; Lapotko, D.O. Influence of transient environmental photothermal effects on optical scattering by gold nanoparticles. Nano Lett. 2009, 9, 2160–2166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lapotko, D. Plasmonic nanoparticle-generated photothermal bubbles and their biomedical applications. Nanomedicine 2009, 4, 813–845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, G.; Antaris, A.L.; Dai, H. Near-infrared fluorophores for biomedical imaging. Nat. Biomed. Eng. 2017, 1, 0010. [Google Scholar] [CrossRef]
- Zhao, H.; Ding, R.; Zhao, X.; Li, Y.; Qu, L.; Pei, H.; Yildirimer, L.; Wu, Z.; Zhang, W. Graphene-based nanomaterials for drug and/or gene delivery, bioimaging, and tissue engineering. Drug Discov. Today 2017, 22, 1302–1317. [Google Scholar] [CrossRef]
- Goenka, S.; Sant, V.; Sant, S. Graphene-based nanomaterials for drug delivery and tissue engineering. J. Control. Release 2014, 173, 75–88. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, S.; Liu, Z. Graphene based gene transfection. Nanoscale 2011, 3, 1252–1257. [Google Scholar] [CrossRef]
- Liu, Z.; Robinson, J.T.; Sun, X.; Dai, H. PEGylated Nanographene Oxide for Delivery of Water-Insoluble Cancer Drugs. J. Am. Chem. Soc. 2008, 130, 10876–10877. [Google Scholar] [CrossRef] [Green Version]
- Boles, M.A.; Ling, D.; Hyeon, T.; Talapin, D.V. The surface science of nanocrystals. Nat. Mater. 2016, 15, 141. [Google Scholar] [CrossRef]
- Walkey, C.D.; Olsen, J.B.; Guo, H.; Emili, A.; Chan, W.C. Nanoparticle size and surface chemistry determine serum protein adsorption and macrophage uptake. J. Am. Chem. Soc. 2012, 134, 2139–2147. [Google Scholar] [CrossRef]
- Robinson, J.T.; Tabakman, S.M.; Liang, Y.; Wang, H.; Sanchez Casalongue, H.; Vinh, D.; Dai, H. Ultrasmall reduced graphene oxide with high near-infrared absorbance for photothermal therapy. J. Am. Chem. Soc. 2011, 133, 6825–6831. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yang, X.; Ren, J.; Qu, K.; Qu, X. Using graphene oxide high near-infrared absorbance for photothermal treatment of Alzheimer’s disease. Adv. Mater. 2012, 24, 1722–1728. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Liu, Z.; Welsher, K.; Robinson, J.T.; Goodwin, A.; Zaric, S.; Dai, H. Nano-graphene oxide for cellular imaging and drug delivery. Nano Res. 2008, 1, 203–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Li, C.; Brans, T.; Harizaj, A.; Van de Steene, S.; De Beer, T.; De Smedt, S.; Szunerits, S.; Boukherroub, R.; Xiong, R.; et al. Surface Functionalization with Polyethylene Glycol and Polyethyleneimine Improves the Performance of Graphene-Based Materials for Safe and Efficient Intracellular Delivery by Laser-Induced Photoporation. Int. J. Mol. Sci. 2020, 21, 1540. https://doi.org/10.3390/ijms21041540
Liu J, Li C, Brans T, Harizaj A, Van de Steene S, De Beer T, De Smedt S, Szunerits S, Boukherroub R, Xiong R, et al. Surface Functionalization with Polyethylene Glycol and Polyethyleneimine Improves the Performance of Graphene-Based Materials for Safe and Efficient Intracellular Delivery by Laser-Induced Photoporation. International Journal of Molecular Sciences. 2020; 21(4):1540. https://doi.org/10.3390/ijms21041540
Chicago/Turabian StyleLiu, Jing, Chengnan Li, Toon Brans, Aranit Harizaj, Shana Van de Steene, Thomas De Beer, Stefaan De Smedt, Sabine Szunerits, Rabah Boukherroub, Ranhua Xiong, and et al. 2020. "Surface Functionalization with Polyethylene Glycol and Polyethyleneimine Improves the Performance of Graphene-Based Materials for Safe and Efficient Intracellular Delivery by Laser-Induced Photoporation" International Journal of Molecular Sciences 21, no. 4: 1540. https://doi.org/10.3390/ijms21041540
APA StyleLiu, J., Li, C., Brans, T., Harizaj, A., Van de Steene, S., De Beer, T., De Smedt, S., Szunerits, S., Boukherroub, R., Xiong, R., & Braeckmans, K. (2020). Surface Functionalization with Polyethylene Glycol and Polyethyleneimine Improves the Performance of Graphene-Based Materials for Safe and Efficient Intracellular Delivery by Laser-Induced Photoporation. International Journal of Molecular Sciences, 21(4), 1540. https://doi.org/10.3390/ijms21041540