Acetate and Butyrate Improve β-cell Metabolism and Mitochondrial Respiration under Oxidative Stress
Abstract
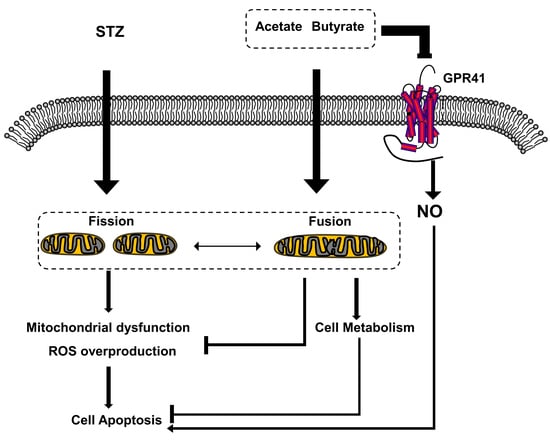
:1. Introduction
2. Results
2.1. SCFAs Influence Human Islet Cell Viability in a Dose-Dependent Manner
2.2. SCFAs Influence β-cell Viability
2.3. SCFAs Prevent Islet Cell-Death During Exposure to STZ
2.4. SCFAs Promote β-Cell Survival in the Presence of STZ
2.5. SCFAs Support β-cell Mitochondrial Respiration and Protect Against STZ-induced β-cell OCR Reduction
2.6. SCFAs Affect β-cells Mitochondrial Dynamics under STZ-Induced Stress
2.7. SCFAs Attenuate STZ-induced Generation of Free Radicals
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Human Islet Isolation
4.3. Cell Viability
4.4. Cell Apoptosis
4.5. Mitochondrial Function
4.6. Reactive Oxygen Species (ROS) Assay
4.7. Nitric Oxide
4.8. Immunofluorescence
4.9. Western Blot
4.10. Quantitative Polymerase Chain Reaction (qPCR)
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cook, S.I.; Sellin, J.H. Review article: Short chain fatty acids in health and disease. Aliment. Pharmacol. Ther. 1998, 12, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Meijer, K.; de Vos, P.; Priebe, M.G. Butyrate and other short-chain fatty acids as modulators of immunity: What relevance for health? Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Mariño, E.; Richards, J.L.; McLeod, K.H.; Stanley, D.; Yap, Y.A.; Knight, J.; McKenzie, C.; Kranich, J.; Oliveira, A.C.; Rossello, F.J.; et al. Gut microbial metabolites limit the frequency of autoimmune T cells and protect against type 1 diabetes. Nat. Immunol. 2017, 18, 552–562. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Wong, F.S. Dietary short-chain fatty acids protect against type 1 diabetes. Nat. Immunol. 2017, 18, 484–486. [Google Scholar] [CrossRef] [Green Version]
- Van der Beek, C.M.; Canfora, E.E.; Kip, A.M.; Gorissen, S.H.M.; Olde Damink, S.W.M.; van Eijk, H.M.; Holst, J.J.; Blaak, E.E.; Dejong, C.H.C.; Lenaerts, K. The prebiotic inulin improves substrate metabolism and promotes short-chain fatty acid production in overweight to obese men. Metabolism 2018, 87, 25–35. [Google Scholar] [CrossRef]
- Canfora, E.E.; van der Beek, C.M.; Jocken, J.W.E.; Goossens, G.H.; Holst, J.J.; Olde Damink, S.W.M.; Lenaerts, K.; Dejong, C.H.C.; Blaak, E.E. Colonic infusions of short-chain fatty acid mixtures promote energy metabolism in overweight/obese men: A randomized crossover trial. Sci. Rep. 2017, 7, 2360. [Google Scholar] [CrossRef]
- Van der Beek, C.M.; Canfora, E.E.; Lenaerts, K.; Troost, F.J.; Damink, S.W.O.M.; Holst, J.J.; Masclee, A.A.M.; Dejong, C.H.C.; Blaak, E.E. Distal, not proximal, colonic acetate infusions promote fat oxidation and improve metabolic markers in overweight/obese men. Clin. Sci. 2016, 130, 2073–2082. [Google Scholar] [CrossRef]
- Canfora, E.E.; Jocken, J.W.; Blaak, E.E. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat. Rev. Endocrinol. 2015, 11, 577–591. [Google Scholar] [CrossRef]
- Hu, J.; Kyrou, I.; Tan, B.K.; Dimitriadis, G.K.; Ramanjaneya, M.; Tripathi, G.; Patel, V.; James, S.; Kawan, M.; Chen, J.; et al. Short-chain fatty acid acetate stimulates adipogenesis and mitochondrial biogenesis via GPR43 in brown adipocytes. Endocrinology 2016, 157, 1881–1894. [Google Scholar] [CrossRef] [Green Version]
- Kimura, I.; Ozawa, K.; Inoue, D.; Imamura, T.; Kimura, K.; Maeda, T.; Terasawa, K.; Kashihara, D.; Hirano, K.; Tani, T.; et al. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat. Commun. 2013, 4, 1829. [Google Scholar] [CrossRef] [Green Version]
- Zaibi, M.S.; Stocker, C.J.; O’Dowd, J.; Davies, A.; Bellahcene, M.; Cawthorne, M.A.; Brown, A.J.H.; Smith, D.M.; Arch, J.R.S. Roles of GPR41 and GPR43 in leptin secretory responses of murine adipocytes to short chain fatty acids. FEBS Lett. 2010, 584, 2381–2386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mollica, M.P.; Mattace Raso, G.; Cavaliere, G.; Trinchese, G.; De Filippo, C.; Aceto, S.; Prisco, M.; Pirozzi, C.; Di Guida, F.; Lama, A.; et al. Butyrate regulates liver mitochondrial function, efficiency, and dynamics in insulin-resistant obese mice. Diabetes 2017, 66, 1405–1418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Z.; Yin, J.; Zhang, J.; Ward, R.E.; Martin, R.J.; Lefevre, M.; Cefalu, W.T.; Ye, J. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 2009, 58, 1509–1517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández, M.A.G.; Canfora, E.E.; Jocken, J.W.E.; Blaak, E.E. The short-chain fatty acid acetate in body weight control and insulin sensitivity. Nutrients 2019, 11, 1943. [Google Scholar] [CrossRef] [Green Version]
- Müller, M.; Hernández, M.A.G.; Goossens, G.H.; Reijnders, D.; Holst, J.J.; Jocken, J.W.E.; van Eijk, H.; Canfora, E.E.; Blaak, E.E. Circulating but not faecal short-chain fatty acids are related to insulin sensitivity, lipolysis and GLP-1 concentrations in humans. Sci. Rep. 2019, 9, 12515. [Google Scholar] [CrossRef] [Green Version]
- Pingitore, A.; Gonzalez-Abuin, N.; Ruz-Maldonado, I.; Huang, G.C.; Frost, G.; Persaud, S.J. Short chain fatty acids stimulate insulin secretion and reduce apoptosis in mouse and human islets in vitro: Role of free fatty acid receptor 2. Diabetes Obes. Metab. 2019, 21, 330–339. [Google Scholar] [CrossRef] [Green Version]
- Priyadarshini, M.; Layden, B.T. FFAR3 modulates insulin secretion and global gene expression in mouse islets. Islets 2015, 7, e1045182. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Fontes, G.; Saxena, G.; Poitout, V.; Shalev, A. Lack of TXNIP protects against mitochondria-mediated apoptosis but not against fatty acid-induced ER stress-mediated β-cell death. Diabetes 2010, 59, 440–447. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Zheng, J. Single-cell gene expression analysis reveals β-cell dysfunction and deficit mechanisms in type 2 diabetes. BMC Bioinform. 2018, 19, 515. [Google Scholar] [CrossRef]
- Detmer, S.A.; Chan, D.C. Functions and dysfunctions of mitochondrial dynamics. Nat. Rev. Mol. Cell Biol. 2007, 8, 870–879. [Google Scholar] [CrossRef]
- Youle, R.J.; Karbowski, M. Mitochondrial fission in apoptosis. Nat. Rev. Mol. Cell Biol. 2005, 6, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Llacua, L.A.; de Haan, B.J.; de Vos, P. Laminin and collagen IV inclusion in immunoisolating microcapsules reduces cytokine-mediated cell death in human pancreatic islets. J. Tissue Eng. Regen. Med. 2018, 12, 460–467. [Google Scholar] [CrossRef]
- Papas, K.K.; Bellin, M.D.; Sutherland, D.E.R.; Suszynski, T.M.; Kitzmann, J.P.; Avgoustiniatos, E.S.; Gruessner, A.C.; Mueller, K.R.; Beilman, G.J.; Balamurugan, A.N.; et al. Islet Oxygen Consumption Rate (OCR) dose predicts insulin independence in clinical islet autotransplantation. PLoS ONE 2015, 10, e0134428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eleazu, C.O.; Eleazu, K.C.; Chukwuma, S.; Essien, U.N. Review of the mechanism of cell death resulting from streptozotocin challenge in experimental animals, its practical use and potential risk to humans. J Diabetes Metab. Disord. 2013, 12, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danobeitia, J.S.; Chlebeck, P.J.; Shokolenko, I.; Ma, X.; Wilson, G.; Fernandez, L.A. Novel fusion protein targeting mitochondrial DNA improves pancreatic islet functional potency and islet transplantation outcomes. Cell Transpl. 2017, 26, 1742–1754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Modak, M.A.; Datar, S.P.; Bhonde, R.R.; Ghaskadbi, S.S. Differential susceptibility of chick and mouse islets to streptozotocin and its co-relation with islet antioxidant status. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 2007, 177, 247–257. [Google Scholar] [CrossRef]
- Wang, R.X.; Li, S.; Sui, X. Sodium butyrate relieves cerebral ischemia-reperfusion injury in mice by inhibiting JNK/STAT pathway. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 1762–1769. [Google Scholar] [CrossRef]
- Jia, Y.; Hong, J.; Li, H.; Hu, Y.; Jia, L.; Cai, D.; Zhao, R. Butyrate stimulates adipose lipolysis and mitochondrial oxidative phosphorylation through histone hyperacetylation-associated β3-adrenergic receptor activation in high-fat diet-induced obese mice. Exp. Physiol. 2017, 102, 273–281. [Google Scholar] [CrossRef] [Green Version]
- Pingitore, A.; Chambers, E.S.; Hill, T.; Maldonado, I.R.; Liu, B.; Bewick, G.; Morrison, D.J.; Preston, T.; Wallis, G.A.; Tedford, C.; et al. The diet-derived short chain fatty acid propionate improves beta-cell function in humans and stimulates insulin secretion from human islets in vitro. Diabetes Obes. Metab. 2017, 19, 257–265. [Google Scholar] [CrossRef] [Green Version]
- Villa, S.R.; Priyadarshini, M.; Fuller, M.H.; Bhardwaj, T.; Brodsky, M.R.; Angueira, A.R.; Mosser, R.E.; Carboneau, B.A.; Tersey, S.A.; Mancebo, H.; et al. Loss of free fatty acid receptor 2 leads to impaired islet mass and beta cell survival. Sci. Rep. 2016, 6, 28159. [Google Scholar] [CrossRef] [Green Version]
- Smink, A.M.; Li, S.; Swart, D.H.; Hertsig, D.T.; de Haan, B.J.; Kamps, J.A.A.M.; Schwab, L.; van Apeldoorn, A.A.; de Koning, E.; Faas, M.M.; et al. Stimulation of vascularization of a subcutaneous scaffold applicable for pancreatic islet-transplantation enhances immediate post-transplant islet graft function but not long-term normoglycemia. J. Biomed. Mater. Res. A 2017, 105, 2533–2542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smelt, M.J.; Faas, M.M.; de Haan, B.J.; de Vos, P. Pancreatic beta-cell purification by altering FAD and NAD(P)H metabolism. Exp. Diabetes Res. 2008, 2008, 165360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goyal, S.N.; Reddy, N.M.; Patil, K.R.; Nakhate, K.T.; Ojha, S.; Patil, C.R.; Agrawal, Y.O. Challenges and issues with streptozotocin-induced diabetes—A clinically relevant animal model to understand the diabetes pathogenesis and evaluate therapeutics. Chem. Biol. Interact. 2016, 244, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Turk, J.; Corbett, J.A.; Ramanadham, S.; Bohrer, A.; McDaniel, M.L. Biochemical evidence for nitric oxide formation from streptozotocin in isolated pancreatic islets. Biochem. Biophys. Res. Commun. 1993, 197, 1458–1464. [Google Scholar] [CrossRef]
- Clark, A.; Mach, N. The crosstalk between the gut microbiota and mitochondria during exercise. Front. Physiol. 2017, 8, 319. [Google Scholar] [CrossRef]
- Xiong, W.; Ma, Z.; An, D.; Liu, Z.; Cai, W.; Bai, Y.; Zhan, Q.; Lai, W.; Zeng, Q.; Ren, H.; et al. Mitofusin 2 participates in mitophagy and mitochondrial fusion against angiotensin II-induced cardiomyocyte injury. Front. Physiol. 2019, 10, 411. [Google Scholar] [CrossRef]
- Duraisamy, A.J.; Mohammad, G.; Kowluru, R.A. Mitochondrial fusion and maintenance of mitochondrial homeostasis in diabetic retinopathy. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 1617–1626. [Google Scholar] [CrossRef]
- Cummings, J.H.; Pomare, E.W.; Branch, W.J.; Naylor, C.P.; Macfarlane, G.T. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 1987, 28, 1221–1227. [Google Scholar] [CrossRef] [Green Version]
- Aoki, R.; Kamikado, K.; Suda, W.; Takii, H.; Mikami, Y.; Suganuma, N.; Hattori, M.; Koga, Y. A proliferative probiotic Bifidobacterium strain in the gut ameliorates progression of metabolic disorders via microbiota modulation and acetate elevation. Sci. Rep. 2017, 7, 43522. [Google Scholar] [CrossRef]
- Koves, T.R.; Ussher, J.R.; Noland, R.C.; Slentz, D.; Mosedale, M.; Ilkayeva, O.; Bain, J.; Stevens, R.; Dyck, J.R.B.; Newgard, C.B.; et al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 2008, 7, 45–56. [Google Scholar] [CrossRef] [Green Version]
- Schönfeld, P.; Więckowski, M.R.; Lebiedzińska, M.; Wojtczak, L. Mitochondrial fatty acid oxidation and oxidative stress: Lack of reverse electron transfer-associated production of reactive oxygen species. Biochim. Biophys. Acta Bioenerg. 2010, 1797, 929–938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, S.; Dong, T.S.; Dalal, S.R.; Wu, F.; Bissonnette, M.; Kwon, J.H.; Chang, E.B. The microbe-derived short chain fatty acid butyrate targets miRNA-dependent p21 gene expression in human colon cancer. PLoS ONE 2011, 6, e16221. [Google Scholar] [CrossRef] [PubMed]
- Siavoshian, S.; Blottiere, H.M.; Cherbut, C.; Galmiche, J.-P. Butyrate stimulates cyclin D and p21 and inhibits cyclin-dependent kinase 2 expression in HT-29 colonic epithelial cells. Biochem. Biophys. Res. Commun. 1997, 232, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Sivaprakasam, S.; Bhutia, Y.D.; Yang, S.; Ganapathy, V. Short-chain fatty acid transporters: Role in colonic homeostasis. Compr. Physiol. 2017, 8, 299–314. [Google Scholar] [CrossRef]
- Hasan, A.U.; Rahman, A.; Kobori, H. Interactions between host PPARs and gut microbiota in health and disease. Int. J. Mol. Sci. 2019, 20, 387. [Google Scholar] [CrossRef] [Green Version]
- Byndloss, M.X.; Olsan, E.E.; Rivera-Chávez, F.; Tiffany, C.R.; Cevallos, S.A.; Lokken, K.L.; Torres, T.P.; Byndloss, A.J.; Faber, F.; Gao, Y.; et al. Microbiota-activated PPAR-γ signaling inhibits dysbiotic Enterobacteriaceae expansion. Science 2017, 357, 570–575. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.P.; Wang, S.; Nagpal, R.; Miller, B.; Singh, R.; Taraphder, S.; Yadav, H. Probiotics and prebiotics for the amelioration of type 1 diabetes: Present and future perspectives. Microorganisms 2019, 7, 67. [Google Scholar] [CrossRef] [Green Version]
- Imai, S.; Fukui, M.; Kajiyama, S. Effect of eating vegetables before carbohydrates on glucose excursions in patients with type 2 diabetes. J. Clin. Biochem. Nutr. 2014, 54, 7–11. [Google Scholar] [CrossRef] [Green Version]
- Weickert, M.O.; Pfeiffer, A.F. Impact of dietary fiber consumption on insulin resistance and the prevention of type 2 diabetes. J. Nutr. 2018, 148, 7–12. [Google Scholar] [CrossRef] [Green Version]
- Ricordi, C.; Lacy, P.E.; Finke, E.H.; Olack, B.J.; Scharp, D.W. Automated method for isolation of human pancreatic islets. Diabetes 1988, 37, 413–420. [Google Scholar] [CrossRef]
- Smink, A.M.; de Haan, B.J.; Paredes-Juarez, G.A.; Wolters, A.H.G.; Kuipers, J.; Giepmans, B.N.G.; Schwab, L.; Engelse, M.A.; van Apeldoorn, A.A.; de Koning, E.; et al. Selection of polymers for application in scaffolds applicable for human pancreatic islet transplantation. Biomed. Mater. 2016, 11, 035006. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Yu, L.-T.; Yang, M.-Q.; Li, X.; Zhang, Z.-Y.; Alfred, M.O.; Liu, J.-L.; Wang, M. Recombinant Reg3β protein protects against streptozotocin-induced β-cell damage and diabetes. Sci. Rep. 2016, 6, 35640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frey, O.; Misun, P.M.; Fluri, D.A.; Hengstler, J.G.; Hierlemann, A. Reconfigurable microfluidic hanging drop network for multi-tissue interaction and analysis. Nat. Commun. 2014, 5, 4250. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Gene | Species | Forward Sequence 5′-3′ | Reverse Sequence 5′-3′ |
|---|---|---|---|
| MFN1 | Mouse | TCTCCAAGCCCAACATCTTCA | ACTCCGGCTCCGAAGCA |
| MFN2 | Mouse | ACAGCCTCAGCCGACAGCAT | TGCCGAAGGAGCAGACCTT |
| DRP1 | Mouse | GCGCTGATCCCGCGTCAT | CCGCACCCACTGTGTTGA |
| OPA1 | Mouse | TGGGCTGCAGAGGATGGT | CCTGATGTCACGGTGTTGATG |
| FIS1 | Mouse | GCCCCTGCTACTGGACCAT | CCCTGAAAGCCTCACACTAAGG |
| β-Actin | Mouse | ACGGCCAGGTCATCACTATTC | AGGAAGGCTGGAAAAGAGCC |
| MFN1 | Human | TGGCTAAGAAGGCGATTACTGC | TCTCCGAGATAGCACCTCACC |
| MFN2 | Human | CTCTCGATGCAACTCTATCGTC | TCCTGTACGTGTCTTCAAGGAA |
| DRP1 | Human | CTGCCTCAAATCGTCGTAGTG | GAGGTCTCCGGGTGACAATTC |
| OPA1 | Human | TGTGAGGTCTGCCAGTCTTTA | TGTCCTTAATTGGGGTCGTTG |
| FIS1 | Human | GTCCAAGAGCACGCAGTTTG | ATGCCTTTACGGATGTCATCATT |
| β-Actin | Human | GCACCACACCTTCTACAATG | TGCTTGCTGATCCACATCTG |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, S.; Kuwabara, R.; de Haan, B.J.; Smink, A.M.; de Vos, P. Acetate and Butyrate Improve β-cell Metabolism and Mitochondrial Respiration under Oxidative Stress. Int. J. Mol. Sci. 2020, 21, 1542. https://doi.org/10.3390/ijms21041542
Hu S, Kuwabara R, de Haan BJ, Smink AM, de Vos P. Acetate and Butyrate Improve β-cell Metabolism and Mitochondrial Respiration under Oxidative Stress. International Journal of Molecular Sciences. 2020; 21(4):1542. https://doi.org/10.3390/ijms21041542
Chicago/Turabian StyleHu, Shuxian, Rei Kuwabara, Bart J. de Haan, Alexandra M. Smink, and Paul de Vos. 2020. "Acetate and Butyrate Improve β-cell Metabolism and Mitochondrial Respiration under Oxidative Stress" International Journal of Molecular Sciences 21, no. 4: 1542. https://doi.org/10.3390/ijms21041542
APA StyleHu, S., Kuwabara, R., de Haan, B. J., Smink, A. M., & de Vos, P. (2020). Acetate and Butyrate Improve β-cell Metabolism and Mitochondrial Respiration under Oxidative Stress. International Journal of Molecular Sciences, 21(4), 1542. https://doi.org/10.3390/ijms21041542