Burkholderia cenocepacia H111 Produces a Water-Insoluble Exopolysaccharide in Biofilm: Structural Determination and Molecular Modelling
Abstract
:1. Introduction
2. Results
2.1. Purification and Composition Analysis of the Water-Insoluble Exopolysaccharide
2.2. NMR Assignments for Epol H111-INS Repeating Unit
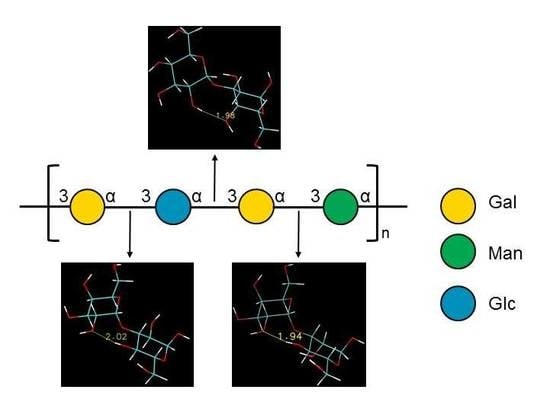
[3)-α-D-Galp-(1→3)-α-D-Glcp-(1→3)-α-D-Galp-(1→3)-α-D-Manp-(1→]n
2.3. Ramachandran Conformational Maps and Molecular Dynamics Simulations
3. Discussion
4. Materials and Methods
4.1. Bacterial Strain, Biofilm Production and Polysaccharide Purification
4.2. General Procedures
4.3. NMR Experiments
4.4. Molecular Modelling
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 1D NMR | One-Dimensional Nuclear Magnetic Resonance |
| 2D NMR | Two-Dimensional Nuclear Magnetic Resonance |
| BCC | Burkholderia Cepacia Complex |
| Bep | Burkholderia cenocepacia exopolysaccharide |
| CF | Cystic fibrosis |
| COSY | Correlation SpectroscopY |
| Epol | Exopolysaccharide |
| Gal | Galactose |
| gHMBCAD | Gradient Heteronuclear Multiple Bond Coherence ADiabatic |
| gHSQCAD | gradient Heteronuclear Single Quantum Coherence ADiabatic |
| Glc | Glucose |
| GLC | Gas–Liquid Chromatography |
| GLC–MS | Gas–Liquid Chromatography–Mass Spectrometry |
| Man | Mannose |
| MD | Molecular dynamics |
| MM | Molecular mechanics |
| NaOD | Deuterated sodium hydroxide |
| NaOH | Sodium hydroxide |
| NOE | Nuclear Overhauser Effect |
| NOESY | Nuclear Overhauser Effect SpectroscopY |
| NYG | Nutrient-yeast extract-glycerol |
| PMAA | Partially methylated alditol acetates |
| RU | repeating unit |
| TFA | Trifluoroacetic acid |
| TMS | Trimethylsilyl |
| TOCSY | TOtal Correlation SpectroscopY |
References
- Drevinek, P.; Mahenthiralingam, E. Burkholderia cenocepacia in cystic fibrosis: Epidemiology and molecular mechanisms of virulence. Clin. Microbiol. Infect. 2010, 16, 821–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loutet, S.A.; Valvano, M.A. A decade of Burkholderia cenocepacia virulence determinant research. Infect. Immun. 2010, 78, 4088–4100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pellizzoni, E.; Ravalico, F.; Scaini, D.; Delneri, A.; Rizzo, R.; Cescutti, P. Biofilms produced by Burkholderia cenocepacia: Influence of media and solid supports on composition of matrix exopolysaccharides. Microbiology 2016, 162, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Costerton, J.W.; Lewandowski, Z.; Caldwell, D.E.; Korber, D.R.; Lappin-Scott, H.M. Microbial biofilms. Annu. Rev. Microbiol. 1995, 49, 711–745. [Google Scholar] [CrossRef]
- Pamp, S.J.; Gjermansen, M.; Tolker-Nielsen, T. The biofilm matrix: A sticky framework. In The Biofilm Mode of Life: Mechanisms and Adaptations; Kjelleberg, S., Givskov, M., Eds.; Horizon Scientific Press: London, UK, 2007; pp. 37–69. [Google Scholar]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Fazli, M.; McCarthy, Y.; Givskov, M.; Ryan, R.P.; Tolker-Nielsen, T. The exopolysaccharide gene cluster Bcam1330–Bcam1341 is involved in Burkholderia cenocepacia biofilm formation, and its expression is regulated by c-di-GMP and Bcam1349. MicrobiologyOpen 2013, 2, 105–122. [Google Scholar] [CrossRef] [Green Version]
- Fazli, M.; Rybtke, M.; Steiner, E.; Weidel, E.; Berthelsen, J.; Groizeleau, J.; Bin, W.; Zhao Zhi, B.; Yaming, Z.; Kaever, V.; et al. Regulation of Burkholderia cenocepacia biofilm formation by RpoN and the c-di-GMP effector BerB. MicrobiologyOpen 2017, 6, e00480. [Google Scholar] [CrossRef] [Green Version]
- Carlier, A.; Agnoli, K.; Pessi, G.; Suppiger, A.; Jenul, C.; Schmid, N.; Tümmler, B.; Pinto-Carbo, M.; Eberl, L. Genome sequence of Burkholderia cenocepacia H111, a cystic fibrosis airway isolate. Genome Announc. 2014, 2, e00298-14. [Google Scholar] [CrossRef] [Green Version]
- Bock, K.; Lundt, I.; Pedersen, C. Assignment of anomeric structure to carbohydrates through germinal 13C-H coupling constants. Tetrahedron Lett. 1973, 14, 1037–1040. [Google Scholar] [CrossRef]
- Jansson, P.E.; Kenne, L.; Widmalm, G. Computer-assisted structural analysis of polysaccharides with an extended version of CASPER using 1H-and 13C-NMR data. Carbohydr. Res. 1989, 188, 169–191. [Google Scholar] [CrossRef]
- Albersheim, P.; Nevins, D.J.; English, P.D.; Karr, A. A method for the analysis of sugars in plant cell-wall polysaccharides by gas-liquid chromatography. Carbohydr. Res. 1967, 5, 340–345. [Google Scholar] [CrossRef]
- Harris, P.J.; Henry, R.J.; Blakeney, A.B.; Stone, B.A. An improved procedure for the methylation analysis of oligosaccharides and polysaccharides. Carbohydr. Res. 1984, 127, 59–73. [Google Scholar] [CrossRef]
- Gerwig, G.J.; Kamerling, J.P.; Vliegenthart, J.F.G. Determination of the D and L configuration of neutral monosaccharides by high-resolution capillary g.l.c. Carbohydr. Res. 1978, 62, 349–357. [Google Scholar] [CrossRef] [Green Version]
- Brooks, B.R.; Bruccoleri, R.E.; Olafson, B.D.; Swaminathan, S.; Karplus, M. CHARMM: A Program for Macromolecular Energy, Minimization, and Dynamics Calculations. J. Comput. Chem. 1983, 4, 187–217. [Google Scholar] [CrossRef]
- Brooks, B.R.; Brooks, C.L.; MacKerell, A.D., Jr.; Nilsson, L.; Petrella, R.J.; Roux, B.; Won, Y.; Archontis, G.; Bartels, C.; Boresch, S.; et al. CHARMM: The biomolecular simulation program. J. Comput. Chem. 2009, 30, 1545–1614. [Google Scholar] [CrossRef] [PubMed]
- Guvench, O.; Greene, S.N.; Kamath, G.; Brady, J.W.; Venable, R.M.; Pastor, R.W.; Mackerell, A.D., Jr. Additive empirical force field for hexopyranose monosaccharides. J. Comput. Chem. 2008, 29, 2543–2564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guvench, O.; Hatcher, E.; Venable, R.M.; Pastor, R.W.; MacKerell, A.D. CHARMM Additive all-atom force field for glycosidic linkages between hexopyranoses. J. Chem. Theory Comput. 2009, 5, 2353–2370. [Google Scholar] [CrossRef] [Green Version]
- Jou, I.A.; Caterino, M.; Schnupf, U.; Rizzo, R.; Cescutti, P.; Brady, J.W. Ramachandran conformational energy maps for disaccharide linkages found in Burkholderia multivorans biofilm polysaccharides. Int. J. Biol. Macromol. 2019, 143, 501–509. [Google Scholar] [CrossRef]
- Fazli, M.; O’Connell, A.; Nilsson, M.; Niehaus, K.; Dow, J.M.; Givskov, M.; Ryan, R.P.; Tolker-Nielsen, T. The CRP/FNR family protein Bcam1349 is a c-di-GMP effector that regulates biofilm formation in the respiratory pathogen Burkholderia cenocepacia. Mol. Microbiol. 2011, 82, 327–341. [Google Scholar] [CrossRef] [PubMed]
- Fazli, M.; Almblad, H.; Rybtke, M.L.; Givskov, M.; Eberl, L.; Tolker-Nielsen, T. Regulation of biofilm formation in Pseudomonas and Burkholderia species. Environ. Microbiol. 2014, 16, 1961–1981. [Google Scholar] [CrossRef]
- Silva, I.N.; Santos, P.M.; Santos, M.R.; Zlosnik, J.E.A.; Speert, D.P.; Buskirk, S.W.; Bruger, E.L.; Waters, C.M.; Cooper, V.S.; Moreira, L.M. Long-term evolution of Burkholderia multivorans during a chronic cystic fibrosis infection reveals shifting forces of selection. mSystems 2016, 1, e00029-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]








| Residue 1 | Chemical Shift (ppm) 2 | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | ||
| A [171] | 1H | 5.29 | 3.89 | 4.02 | 4.20 | 4.14 | 3.89 |
| →3)-α-D-Galp-(1→ | 13C | 101.0 | 68.4 | 74.1 | 66.1 | 72.3 | 62.2 |
| B [171] | 1H | 5.25 | 3.95 | 3.98 | 4.18 | 4.05 | 3.71 |
| →3)-α-D-Galp-(1→ | 13C | 101.6 | 68.2 | 75.5 | 66.6 | 72.4 | 62.3 |
| C [170] | 1H | 5.08 | 3.62 | 3.89 | 3.50 | 3.91 | 3.83 |
| →3)-α-D-Glcp-(1→ | 13C | 96.1 | 71.4 | 82.9 | 71.2 | 73.6 | 62.0 |
| D [169] | 1H | 4.98 | 4.14 | 3.98 | 3.76 | 3.94 | 3.66 |
| →3)-α-D-Manp-(1→ | 13C | 96.9 | 71.1 | 80.4 | 67.5 | 73.9 | 62.1 |
| Proton/ppm. | NOE Contacts (ppm) | Assignment |
|---|---|---|
| A1/5.29 | 3.89 | A2 |
| 3.89 | C3 | |
| B1/5.25 | 3.95 | B2 |
| 3.98 | D3 | |
| C1/5.08 | 3.62 | C2 |
| 3.98 | B3 | |
| 4.18 | B4 | |
| D1/4.98 | 4.14 | D2 |
| 4.02 | A3 | |
| 4.20 | A4 |
| Disaccharide | Highest Density 1 | Average Distance (Å) 2 | |||||
|---|---|---|---|---|---|---|---|
| Vacuum (ф, ψ) | TIP4P (ф, ψ) | H1-‘H3 | H1-‘H4 | H1-‘H5 | H2-‘O2 | H1-‘C3 | |
| α-d-Galp-(1→3)-α-d-Glcp | 94 | -135 | 82 | -137 | 2.38 (0.33) | 3.79 (0.40) | 4.38 (0.30) | 5.68 (0.71) | 2.57 (0.12) |
| α-d-Glcp-(1→3)-α-d-Galp | 99 | -160 | 67 | -162 | 2.73 (0.34) | 2.39 (0.41) | 4.28 (0.25) | 5.60 (0.74) | 2.63 (0.12) |
| α-d-Galp-(1→3)-α-d-Manp | 94 | -136 | 69 | -130 | 2.34 (0.21) | 3.97 (0.31) | 4.40 (0.27) | 5.41 (0.78) | 2.58 (0.10) |
| α-d-Manp-(1→3)-α-d-Galp | 68 | -160 | 60 | -174 | 2.75 (0.33) | 2.38 (0.39) | 4.30 (0.25) | 6.61 (0.32) | 2.64 (0.12) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellich, B.; Jou, I.A.; Caterino, M.; Rizzo, R.; Ravenscroft, N.; Fazli, M.; Tolker-Nielsen, T.; Brady, J.W.; Cescutti, P. Burkholderia cenocepacia H111 Produces a Water-Insoluble Exopolysaccharide in Biofilm: Structural Determination and Molecular Modelling. Int. J. Mol. Sci. 2020, 21, 1702. https://doi.org/10.3390/ijms21051702
Bellich B, Jou IA, Caterino M, Rizzo R, Ravenscroft N, Fazli M, Tolker-Nielsen T, Brady JW, Cescutti P. Burkholderia cenocepacia H111 Produces a Water-Insoluble Exopolysaccharide in Biofilm: Structural Determination and Molecular Modelling. International Journal of Molecular Sciences. 2020; 21(5):1702. https://doi.org/10.3390/ijms21051702
Chicago/Turabian StyleBellich, Barbara, Ining A. Jou, Marco Caterino, Roberto Rizzo, Neil Ravenscroft, Mustafa Fazli, Tim Tolker-Nielsen, John W. Brady, and Paola Cescutti. 2020. "Burkholderia cenocepacia H111 Produces a Water-Insoluble Exopolysaccharide in Biofilm: Structural Determination and Molecular Modelling" International Journal of Molecular Sciences 21, no. 5: 1702. https://doi.org/10.3390/ijms21051702
APA StyleBellich, B., Jou, I. A., Caterino, M., Rizzo, R., Ravenscroft, N., Fazli, M., Tolker-Nielsen, T., Brady, J. W., & Cescutti, P. (2020). Burkholderia cenocepacia H111 Produces a Water-Insoluble Exopolysaccharide in Biofilm: Structural Determination and Molecular Modelling. International Journal of Molecular Sciences, 21(5), 1702. https://doi.org/10.3390/ijms21051702