Polymer Stabilized Cholesteric Liquid Crystal Siloxane for Temperature-Responsive Photonic Coatings
Abstract
:1. Introduction
2. Results
2.1. Synthesis and Characterization of the CLC Oligosiloxane Mixture
2.2. Fabrication of Polymer Stabilized Cholesteric Liquid Crystal Siloxane Coating
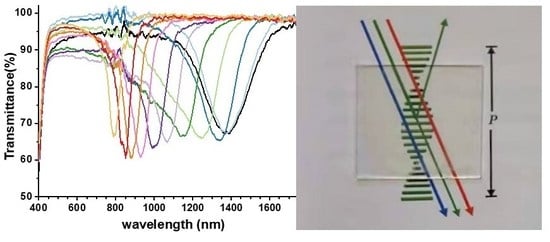
2.3. Temperature Response of the Polymer Stabilized Ch-LC Siloxane Coating
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Mixtures
4.3. Preparation of Rubbed Polyimide Coated Glass Substrates
4.4. Preparation of Coatings
4.5. Characterization
4.6. Polymeric Distribution Analysis of the Coatings
4.7. Vis–NIR Transmission Spectra of the Coatings Over Temperature
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Ch | Cholesteric |
| Sm | Smectic |
| LC | Liquid crystal |
| RM | Reactive mesogens |
| PSLC | Polymer-stabilized liquid crystals |
References
- Stumpel, J.E.; Broer, D.J.; Schenning, A.P.H.J. Stimuli-responsive photonic polymer coatings. Chem. Commun. 2014, 50, 15839–15848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, R.; Chini, A.; Srinivasan, R.S.; Jiang, P. Energy efficiency of smart windows made of photonic crystal. Int. J. Constr. Manag. 2017, 17, 100–112. [Google Scholar] [CrossRef]
- Khandelwal, H.; Schenning, A.P.H.J.; Debije, M.G. Infrared Regulating Smart Window Based on Organic Materials. Adv. Energy Mater. 2017, 7, 1602209. [Google Scholar] [CrossRef]
- Balamurugan, R.; Liu, J.H. A review of the fabrication of photonic band gap materials based on cholesteric liquid crystals. React. Funct. Polym. 2016, 105, 9–34. [Google Scholar] [CrossRef]
- Eelkema, R.; Feringa, B.L. Amplification of chirality in liquid crystals. Org. Biomol. Chem. 2006, 4, 3729–3745. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Mishima, K.; Matsuyama, K.; Hayashi, K.I.; Kikuchi, H.; Kajiyama, T. Thermally bandwidth-controllable reflective polarizers from (polymer network/liquid crystal/chiral dopant) composites. Appl. Phys. Lett. 2003, 82, 2407–2409. [Google Scholar] [CrossRef]
- Yuan, X.; Zhang, L.; Yang, H. Study of selectively reflecting characteristics of polymer stabilised chiral nematic liquid crystal films with a temperature-dependent pitch length. Liq. Cryst. 2010, 37, 445–451. [Google Scholar] [CrossRef]
- Guo, R.; Li, K.; Cao, H.; Wu, X.; Wang, G.; Cheng, Z.; Wang, F.; Zhang, H.; Yang, H. Chiral polymer networks with a broad reflection band achieved with varying temperature. Polymer (Guildf) 2010, 51, 5990–5996. [Google Scholar] [CrossRef]
- Natarajan, L.V.; Wofford, J.M.; Tondiglia, V.P.; Sutherland, R.L.; Koerner, H.; Vaia, R.A.; Bunning, T.J. Electro-thermal tuning in a negative dielectric cholesteric liquid crystal material. J. Appl. Phys. 2008, 103, 6. [Google Scholar] [CrossRef]
- Wu, X.; Cao, H.; Guo, R.; Li, K.; Wang, F.; Yang, H. Effect of cholesteric liquid crystalline elastomer with binaphthalene crosslinkings on thermal and optical properties of a liquid crystal that show smectic A-cholesteric phase transition. Polym. Adv. Technol. 2013, 24, 228–235. [Google Scholar] [CrossRef]
- Vijayaraghavan, R.K.; Abraham, S.; Akiyama, H.; Furumi, S.; Tamaoki, N.; Das, S. Photoresponsive glass-forming butadiene-based chiral liquid crystals with circularly polarized photoluminescence. Adv. Funct. Mater. 2008, 18, 2510–2517. [Google Scholar] [CrossRef]
- Tzeng, S.Y.T.; Chen, C.N.; Tzeng, Y. Thermal tuning band gap in cholesteric liquid crystals. Liq. Cryst. 2010, 37, 1221–1224. [Google Scholar] [CrossRef]
- van Heeswijk, E.; Kragt, A.J.J.; Grossiord, N.; Schenning, A. Environmentally Responsive Photonic Polymers. Chem. Commun. 2019, 55, 2880–2891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, P.; Kragt, A.J.J.; Schenning, A.P.H.J.; De Haan, L.T.; Zhou, G. An easily coatable temperature responsive cholesteric liquid crystal oligomer for making structural colour patterns. J. Mater. Chem. C 2018, 6, 7184–7187. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, M.; Wang, L.; Yang, D.K.; Yu, H.; Yang, H. Polymeric infrared reflective thin films with ultra-broad bandwidth. Liq. Cryst. 2016, 43, 750–757. [Google Scholar] [CrossRef]
- Zhang, B.; Lin, X.; You, Y.; Hu, X.; de Haan, L.; Zhao, W.; Zhou, G.; Yuan, D. Flexible thermal responsive infrared reflector based on cholesteric liquid crystals and polymer stabilized cholesteric liquid crystals. Opt. Express 2019, 27, 13516. [Google Scholar] [CrossRef]
- Khandelwal, H.; van Heeswijk, E.P.A.; Schenning, A.P.H.J.; Debije, M.G. Paintable temperature-responsive cholesteric liquid crystal reflectors encapsulated on a single flexible polymer substrate. J. Mater. Chem. C 2019. [Google Scholar] [CrossRef] [Green Version]
- Ranjkesh, A.; Yoon, T.-H. Fabrication of a Single-Substrate Flexible Thermoresponsive Cholesteric Liquid-Crystal Film with Wavelength Tunability. ACS Appl. Mater. Interfaces 2019, 11, 26314–26322. [Google Scholar] [CrossRef]
- van Heeswijk, E.; Meerman, T.; de Heer, J.; Grossiord, N.; Schenning, A.P.H.J. Paintable encapsulated body-temperature-responsive photonic reflectors with arbitrary shapes. ACS Appl. Polym. Mater. 2019, 1, 3407–3412. [Google Scholar] [CrossRef]
- Van Heeswijk, E.P.A.; Kloos, J.J.H.; Grossiord, N.; Schenning, A.P.H.J. Humidity-gated, temperature-responsive photonic infrared reflective broadband coatings. J. Mater. Chem. A 2019, 7, 6113–6119. [Google Scholar] [CrossRef] [Green Version]
- Nagai, H.; Urayama, K. Thermal response of cholesteric liquid crystal elastomers. Phys. Rev. E 2015, 92, 022501. [Google Scholar] [CrossRef]
- Brannum, M.T.; Steele, A.M.; Venetos, M.C.; Korley, L.S.T.J.; Wnek, G.E.; White, T.J. Light Control with Liquid Crystalline Elastomers. Adv. Opt. Mater. 2019, 1801683, 1–7. [Google Scholar] [CrossRef]
- Wu, X.; Cao, H.; Guo, R.; Li, K.; Wang, F.; Gao, Y.; Yao, W.; Zhang, L.; Chen, X.; Yang, H. Influence of interim alkyl chain length on phase transitions and wide-band reflective behaviors of side-chain liquid crystalline elastomers with binaphthalene crosslinkings. Macromolecules 2012, 45, 5556–5566. [Google Scholar] [CrossRef]
- Wang, J.W.; Zhang, B.Y. Synthesis and optical properties of cholesteric liquid-crystalline oligomers displaying reversible thermochromism. J. Appl. Polym. Sci. 2013, 130, 1321–1327. [Google Scholar] [CrossRef]
- Zhang, W.; Kragt, S.; Schenning, A.P.H.J.; De Haan, L.T.; Zhou, G. Easily Processable Temperature-Responsive Infrared-Reflective Polymer Coatings. ACS Omega 2017, 2, 3475–3482. [Google Scholar] [CrossRef]
- Li, S.Y.; Niklasson, G.A.; Granqvist, C.G. Thermochromic fenestration with VO2-based materials: Three challenges and how they can be met. Thin Solid Films 2012, 520, 3823–3828. [Google Scholar] [CrossRef]
- Ke, Y.; Chen, J.; Lin, G.; Wang, S.; Zhou, Y.; Yin, J.; Lee, P.S.; Long, Y. Smart Windows: Electro-, Thermo-, Mechano-, Photochromics, and Beyond. Adv. Energy Mater. 2019, 9, 1902066. [Google Scholar] [CrossRef]
- Liang, X.; Guo, S.S.; Guo, S.S.; Chen, M.; Li, C.; Wang, Q.; Zou, C.; Zhang, C.; Zhang, L.; Yang, H. A temperature and electric field-responsive flexible smart film with full broadband optical modulation. Mater. Horizons 2017, 4, 878–884. [Google Scholar] [CrossRef]
- Liang, X.; Guo, C.; Chen, M.; Guo, S.; Zhang, L.; Li, F.; Guo, S.; Yang, H. A roll-to-roll process for multi-responsive soft-matter composite films containing Cs:XWO3nanorods for energy-efficient smart window applications. Nanoscale Horizons 2017, 2, 319–325. [Google Scholar] [CrossRef]
- Zhou, Y.; Cai, Y.; Hu, X.; Long, Y. Temperature-responsive hydrogel with ultra-large solar modulation and high luminous transmission for “smart window” applications. J. Mater. Chem. A 2014, 2, 13550–13555. [Google Scholar] [CrossRef]
- Zhou, Y.; Cai, Y.; Hu, X.; Long, Y. VO2/hydrogel hybrid nanothermochromic material with ultra-high solar modulation and luminous transmission. J. Mater. Chem. A 2015, 3, 1121–1126. [Google Scholar] [CrossRef]
- Stevens, H.; Rehage, G.; Finkelmann, H. Phase Transformations of Liquid Crystalline Side-Chain Oligomers. Macromolecules 1984, 17, 851–856. [Google Scholar] [CrossRef]
- Finkelmann, H.; Rehage, G. Investigations on liquid crystalline polysiloxanes, 2. Optical properties of cholesteric phase and influence of the flexible spacer on the mobility of the mesogenic group. Macromol. Chem. Rapid Commun. 1980, 1, 733–740. [Google Scholar] [CrossRef]
- Lacey, D.; Beattie, H.N.; Mitchell, G.R.; Pople, J.A. Orientation effects in monodomain nematic liquid crystalline polysiloxane elastomers. J. Mater. Chem. 1998, 8, 53–60. [Google Scholar] [CrossRef]
- Nishihama, S.; Yamada, H.; Nakazawa, H. Polymerization of tetramethylcyclotetrasiloxane monomer by ion- exchanged montmorillonite catalysts. Clay Miner. 1997, 32, 645–651. [Google Scholar] [CrossRef]
- Kragt, A.J.J.; Broer, D.J.; Schenning, A.P.H.J. Easily Processable and Programmable Responsive Semi-Interpenetrating Liquid Crystalline Polymer Network Coatings with Changing Reflectivities and Surface Topographies. Adv. Funct. Mater. 2018, 28, 1704756. [Google Scholar] [CrossRef] [Green Version]
- Van Meter, J.P.; Klanderman, B.H. Mesomorphic Properties of Some Phenyl Benzoate Dericatives. Mol. Cryst. Liq. Cryst. 1973, 22, 271–284. [Google Scholar] [CrossRef]
- Lecamp, L.; Youssef, B.; Bunel, C.; Lebaudy, P. Photoinitiated polymerization of a dimethacrylate oligomer: 1. Influence of photoinitiator concentration, temperature and light intensity. Polymer (Guildf) 1997, 38, 6089–6096. [Google Scholar] [CrossRef]
- Karausta, A.; Bukusoglu, E. Liquid Crystal-Templated Synthesis of Mesoporous Membranes with Predetermined Pore Alignment. ACS Appl. Mater. Interfaces 2018, 10, 33484–33492. [Google Scholar] [CrossRef]
- Finkelmann, H.; Rehage, G. Liquid Crystal Side Chain Polymers. Adv. Polym. Sci. 1984, 60/61, 99–172. [Google Scholar]
- Sonin, A.S.; Churochkina, N.A. Liquid crystals stabilized by polymer networks. Polym. Sci. Ser. A 2010, 52, 463–482. [Google Scholar] [CrossRef]
- Rajaram, C.V.; Hudson, S.D.; Chien, L.C. Morphology of Polymer-Stabilized Liquid Crystals. Chem. Mater. 1995, 7, 2300–2308. [Google Scholar] [CrossRef]
- Kragt, A.J.J.; Hoekstra, D.C.; Stallinga, S.; Broer, D.J.; Schenning, A.P.H.J. 3D Helix Engineering in Chiral Photonic Materials. Adv. Mater. 2019, 31, 1903120. [Google Scholar] [CrossRef]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Lub, J.; Schenning, A.P.H.J.; Zhou, G.; de Haan, L.T. Polymer Stabilized Cholesteric Liquid Crystal Siloxane for Temperature-Responsive Photonic Coatings. Int. J. Mol. Sci. 2020, 21, 1803. https://doi.org/10.3390/ijms21051803
Zhang W, Lub J, Schenning APHJ, Zhou G, de Haan LT. Polymer Stabilized Cholesteric Liquid Crystal Siloxane for Temperature-Responsive Photonic Coatings. International Journal of Molecular Sciences. 2020; 21(5):1803. https://doi.org/10.3390/ijms21051803
Chicago/Turabian StyleZhang, Weixin, Johan Lub, Albertus P.H.J. Schenning, Guofu Zhou, and Laurens T. de Haan. 2020. "Polymer Stabilized Cholesteric Liquid Crystal Siloxane for Temperature-Responsive Photonic Coatings" International Journal of Molecular Sciences 21, no. 5: 1803. https://doi.org/10.3390/ijms21051803
APA StyleZhang, W., Lub, J., Schenning, A. P. H. J., Zhou, G., & de Haan, L. T. (2020). Polymer Stabilized Cholesteric Liquid Crystal Siloxane for Temperature-Responsive Photonic Coatings. International Journal of Molecular Sciences, 21(5), 1803. https://doi.org/10.3390/ijms21051803