Photochemical Printing of Plasmonically Active Silver Nanostructures
Abstract
:1. Introduction
2. Results and Discussion
2.1. Photoactive Protein
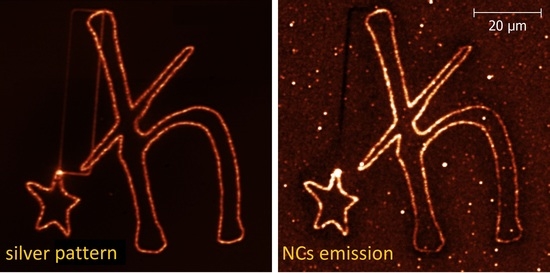
2.2. Up-Converting Nanocrystals
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bharadwaj, P.; Anger, P.; Novotny, L. Nanoplasmonic enhancement of single-molecule fluorescence. Nanotechnology 2007, 18, 044017. [Google Scholar] [CrossRef]
- Mauser, N.; Piatkowski, D.; Mancabelli, T.; Nyk, M.; Mackowski, S.; Hartschuh, A. Tip Enhancement of Upconversion Photoluminescence from Rare Earth Ion Doped Nanocrystals. ACS Nano 2015, 9, 3617–3626. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, P.; Novotny, L. Spectral dependence of single molecule fluorescence enhancement. Opt. Express 2007, 15, 14266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiley, B.J.; Im, S.H.; Li, Z.-Y.; McLellan, J.; Siekkinen, A.; Xia, Y. Maneuvering the Surface Plasmon Resonance of Silver Nanostructures through Shape-Controlled Synthesis. J. Phys. Chem. B 2006, 110, 15666–15675. [Google Scholar] [CrossRef] [PubMed]
- Eustis, S.; El-Sayed, M.A. Why gold nanoparticles are more precious than pretty gold: Noble metal surface plasmon resonance and its enhancement of the radiative and nonradiative properties of nanocrystals of different shapes. Chem. Soc. Rev. 2006, 35, 209–217. [Google Scholar] [CrossRef]
- Giannini, V.; Fernández-Domínguez, A.I.; Heck, S.C.; Maier, S.A. Plasmonic Nanoantennas: Fundamentals and Their Use in Controlling the Radiative Properties of Nanoemitters. Chem. Rev. 2011, 111, 3888–3912. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: Amsterdam, The Netherland, 2006; ISBN 978-0-387-31278-1. [Google Scholar]
- Aslan, K.; Leonenko, Z.; Lakowicz, J.R.; Geddes, C.D. Annealed silver-island films for applications in metal-enhanced fluorescence: Interpretation in terms of radiating plasmons. J. Fluoresc. 2005, 15, 643–654. [Google Scholar] [CrossRef] [Green Version]
- Ciszak, K.K.; Olejnik, M.; Strzelecki, J.; Krajnik, B.; Piątkowski, D.; Hofmann, E.; Mackowski, S. Influence of Plasmon Resonance in Silver Island Film on the Optical Properties of Peridinin-Chlorophyll-Protein Light-Harvesting Complexes. Acta Phys. Pol. A 2012, 122, 275–278. [Google Scholar] [CrossRef]
- Ray, K.; Badugu, R.; Lakowicz, J.R. Metal-Enhanced Fluorescence from CdTe Nanocrystals: A Single-Molecule Fluorescence Study. J. Am. Chem. Soc. 2006, 128, 8998–8999. [Google Scholar] [CrossRef] [Green Version]
- Czechowski, N.; Lokstein, H.; Kowalska, D.; Ashraf, K.; Cogdell, R.J.; Mackowski, S. Large plasmonic fluorescence enhancement of cyanobacterial photosystem I coupled to silver island films. Appl. Phys. Lett. 2014, 105, 043701. [Google Scholar] [CrossRef]
- Turrell, G.; Corset, J. Raman Microscopy: Developments and Applications; Academic Press: Cambridge, MA, USA, 1996; ISBN 978-0-08-054025-2. [Google Scholar]
- Staiano, M.; Matveeva, E.G.; Rossi, M.; Crescenzo, R.; Gryczynski, Z.; Gryczynski, I.; Iozzino, L.; Akopova, I.; D’Auria, S. Nanostructured Silver-Based Surfaces: New Emergent Methodologies for an Easy Detection of Analytes. ACS Appl. Mater. Interfaces 2009, 1, 2909–2916. [Google Scholar] [CrossRef]
- Geddes, C.D.; Parfenov, A.; Lakowicz, J.R. Photodeposition of Silver Can Result in Metal-Enhanced Fluorescence. Appl. Spectrosc. 2003, 57, 526–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, K.; Spendier, K.; Pinchuk, A.O. Laser-Directed Deposition of Silver Nanostructures; Boardman, A.D., Ed.; SPIE: Washington, DC, USA, 2014; p. 916314. [Google Scholar]
- Kleima, F.J.; Wendling, M.; Hofmann, E.; Peterman, E.J.G.; van Grondelle, R.; van Amerongen, H. Peridinin Chlorophyll a Protein: Relating Structure and Steady-State Spectroscopy. Biochemistry 2000, 39, 5184–5195. [Google Scholar] [CrossRef]
- Mackowski, S. Hybrid nanostructures for efficient light harvesting. J. Phys. Condens. Matter 2010, 22, 193102. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Hao, S.; Yang, C.; Chen, G. Enhancing Solar Cell Efficiency Using Photon Upconversion Materials. Nanomaterials 2015, 5, 1782–1809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olejnik, M.; Krajnik, B.; Kowalska, D.; Twardowska, M.; Czechowski, N.; Hofmann, E.; Mackowski, S. Imaging of fluorescence enhancement in photosynthetic complexes coupled to silver nanowires. Appl. Phys. Lett. 2013, 102, 083703. [Google Scholar] [CrossRef]
- Szalkowski, M.; Olmos, J.D.J.; Buczyńska, D.; Maćkowski, S.; Kowalska, D.; Kargul, J. Plasmon-induced absorption of blind chlorophylls in photosynthetic proteins assembled on silver nanowires. Nanoscale 2017, 9, 10475–10486. [Google Scholar] [CrossRef]
- Anger, P.; Bharadwaj, P.; Novotny, L. Enhancement and quenching of single-molecule fluorescence. Phys. Rev. Lett. 2006, 96, 113002. [Google Scholar] [CrossRef] [Green Version]
- Purcell, E.M. Spontaneous Emission Probabilities at Radio Frequencies. Phys. Rev. 1946, 69, 681. [Google Scholar]
- Barnes, W. Fluorescence near interfaces: The role of photonic mode density. J. Mod. Opt. 1998, 45, 661–699. [Google Scholar] [CrossRef]
- Kumar, R.; Nyk, M.; Ohulchanskyy, T.Y.; Flask, C.A.; Prasad, P.N. Combined Optical and MR Bioimaging Using Rare Earth Ion Doped NaYF4 Nanocrystals. Adv. Funct. Mater. 2009, 19, 853–859. [Google Scholar] [CrossRef]
- Piatkowski, D.; Hartmann, N.; Macabelli, T.; Nyk, M.; Mackowski, S.; Hartschuh, A. Silver nanowires as receiving-radiating nanoantennas in plasmon-enhanced up-conversion processes. Nanoscale 2015, 7, 1479–1484. [Google Scholar] [CrossRef] [PubMed]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szalkowski, M.; Sulowska, K.; Jönsson-Niedziółka, M.; Wiwatowski, K.; Niedziółka-Jönsson, J.; Maćkowski, S.; Piątkowski, D. Photochemical Printing of Plasmonically Active Silver Nanostructures. Int. J. Mol. Sci. 2020, 21, 2006. https://doi.org/10.3390/ijms21062006
Szalkowski M, Sulowska K, Jönsson-Niedziółka M, Wiwatowski K, Niedziółka-Jönsson J, Maćkowski S, Piątkowski D. Photochemical Printing of Plasmonically Active Silver Nanostructures. International Journal of Molecular Sciences. 2020; 21(6):2006. https://doi.org/10.3390/ijms21062006
Chicago/Turabian StyleSzalkowski, Marcin, Karolina Sulowska, Martin Jönsson-Niedziółka, Kamil Wiwatowski, Joanna Niedziółka-Jönsson, Sebastian Maćkowski, and Dawid Piątkowski. 2020. "Photochemical Printing of Plasmonically Active Silver Nanostructures" International Journal of Molecular Sciences 21, no. 6: 2006. https://doi.org/10.3390/ijms21062006
APA StyleSzalkowski, M., Sulowska, K., Jönsson-Niedziółka, M., Wiwatowski, K., Niedziółka-Jönsson, J., Maćkowski, S., & Piątkowski, D. (2020). Photochemical Printing of Plasmonically Active Silver Nanostructures. International Journal of Molecular Sciences, 21(6), 2006. https://doi.org/10.3390/ijms21062006