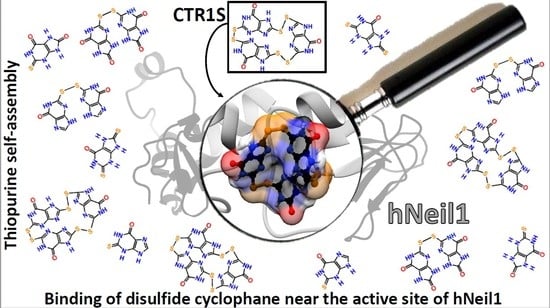
Thiopurine Derivative-Induced Fpg/Nei DNA Glycosylase Inhibition: Structural, Dynamic and Functional Insights
Abstract
:1. Introduction
2. Results and Discussion
2.1. Inhibition of the Bacterial Fpg Activity by 2TX Derivatives
2.2. Inhibition of the Bacterial Fpg DNA Binding Activity by 2TX and TXn
2.3. Selective Inhibition of ZnLF-Containing Fpg/Nei DNA Glycosylases by Dithio-TXn
2.4. Molecular Mechanisms for Fpg/Nei Inhibition by Disulfide forms of 2TX and TXn
2.5. Structural Insights into the Mechanism of Fpg/Nei DNA Glycosylase Inhibition by 2TX and TXn
2.5.1. Proposed Binding Sites from Blind and Flexible Docking of Reduced and Oxidized Forms of 2TX and TX19 on Free and DNA Bound LlFpg and hNeil1
2.5.2. Crystal Structures of Thiopurine Derivatives Bound to Fpg/DNA Complex Partially Confirm Docking Experiments
3. Materials and Methods
3.1. Chemicals and 2TX Derivatives Mini-Library
3.2. Enzymes and DNA Probes
3.3. Enzymes Assays for Inhibition and IC50 Determination
3.4. Molecular Dynamic Simulation and Docking
3.5. Crystallization and X-Ray Structure Determination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| EMSA | Electrophoretic mobility shift assay |
| 2TX | 2-thioxanthine |
| TXn | 2TX derivatives |
| LRC | Lesion recognition complex |
| BER | Base excision repair |
| LCL | Lesion capping loop |
| DMSO | Dimethylsulfoxide |
| THF | Tetrahydrofuran |
| TCEP | Tris(2-carboxyethyl)phosphine hydrochloride |
| DTT | Dithiothreitol |
| 8-oxoG | 8-oxoguanine |
| AP site | Abasic site |
| ZnF | Zinc Finger |
| ZnLF | Zincless Finger |
References
- Lindahl, T. Instability and decay of the primary structure of DNA. Nature 1993, 362, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Cadet, J.; Davies, K.J.A.; Medeiros, M.H.; Di Mascio, P.; Wagner, J.R. Formation and repair of oxidatively generated damage in cellular DNA. Free Radic. Biol. Med. 2017, 107, 13–34. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.C.; Cahill, D.S.; Kasai, H.; Nishimura, S.; Loeb, L.A. 8-Hydroxyguanine, an abundant form of oxidative DNA damage, causes G----T and A----C substitutions. J. Biol. Chem. 1992, 267, 166–172. [Google Scholar] [PubMed]
- Schaaper, R.M.; Kunkel, T.A.; Loeb, L.A. Infidelity of DNA synthesis associated with bypass of apurinic sites. Proc. Natl. Acad. Sci. USA 1983, 80, 487–491. [Google Scholar] [CrossRef] [Green Version]
- Basu, A.K. DNA Damage, Mutagenesis and Cancer. Int. J. Mol. Sci. 2018, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czarny, P.; Wigner, P.; Galecki, P.; Sliwinski, T. The interplay between inflammation, oxidative stress, DNA damage, DNA repair and mitochondrial dysfunction in depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 80, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Leandro, G.S.; Sykora, P.; Bohr, V.A. The impact of base excision DNA repair in age-related neurodegenerative diseases. Mutat. Res. 2015, 776, 31–39. [Google Scholar] [CrossRef] [Green Version]
- Sahan, A.Z.; Hazra, T.K.; Das, S. The Pivotal Role of DNA Repair in Infection Mediated-Inflammation and Cancer. Front. Microbiol. 2018, 9, 663. [Google Scholar] [CrossRef] [Green Version]
- Scharer, O.D. Chemistry and biology of DNA repair. Angew. Chem. 2003, 42, 2946–2974. [Google Scholar] [CrossRef]
- Krokan, H.E.; Bjoras, M. Base excision repair. Cold Spring Harb. Perspect. Biol. 2013, 5, a012583. [Google Scholar] [CrossRef]
- Wallace, S.S. Base excision repair: A critical player in many games. DNA Repair 2014, 19, 14–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Budworth, H.; Harris, F.R.; Williams, P.; Lee, D.Y.; Holt, A.; Pahnke, J. Suppression of Somatic Expansion Delays the Onset of Pathophysiology in a Mouse Model of Huntington’s Disease. PLoS Genet. 2015, 11, e1005267. [Google Scholar] [CrossRef] [PubMed]
- Goula, A.V.; Berquist, B.R.; Wilson, D.M., 3rd; Wheeler, V.C.; Trottier, Y.; Merienne, K. Stoichiometry of base excision repair proteins correlates with increased somatic CAG instability in striatum over cerebellum in Huntington’s disease transgenic mice. PLoS Genet. 2009, 5, e1000749. [Google Scholar] [CrossRef] [PubMed]
- Goula, A.V.; Goula, A.V.; Stys, A.; Chan, J.P.; Trottier, Y.; Festenstein, R.; Merienne, K. Transcription elongation and tissue-specific somatic CAG instability. PLoS Genet. 2012, 8, e1003051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kovtun, I.V.; Liu, Y.; Bjoras, M.; Klungland, A.; Wilson, S.H.; McMurray, C.T. OGG1 initiates age-dependent CAG trinucleotide expansion in somatic cells. Nature 2007, 447, 447–452. [Google Scholar] [CrossRef] [Green Version]
- Taricani, L.; Shanahan, F.; Pierce, R.H.; Guzi, T.J.; Parry, D. Phenotypic enhancement of thymidylate synthetase pathway inhibitors following ablation of Neil1 DNA glycosylase/lyase. Cell Cycle 2010, 9, 4876–4883. [Google Scholar] [CrossRef] [Green Version]
- Ramdzan, Z.M.; Ginjala, V.; Pinder, J.B.; Chung, D.; Donovan, C.M.; Kaur, S.; Leduy, L.; Dellaire, G.; Ganesan, S.; Nepveu, A. The DNA repair function of CUX1 contributes toradioresistance. Oncotarget 2017. [Google Scholar] [CrossRef] [Green Version]
- Biela, A.; Coste, F.; Culard, F.; Guerin, M.; Goffinont, S.; Gasteiger, K.; Cieśla, J.; Winczura, A.; Kazimierczuk, Z.; Gasparutto, D.; et al. Zinc finger oxidation of Fpg/Nei DNA glycosylases by 2-thioxanthine: Biochemical and X-ray structural characterization. Nucleic Acids Res. 2014, 42, 10748–10761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donley, N.; Jaruga, P.; Coskun, E.; Dizdaroglu, M.; McCullough, A.K.; Lloyd, R.S. Small Molecule Inhibitors of 8-Oxoguanine DNA Glycosylase-1 (OGG1). ACS Chem. Biol. 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacobs, A.C.; Calkins, M.J.; Jadhav, A.; Dorjsuren, D.; Maloney, D.; Simeonov, A.; Jaruga, P.; Dizdaroglu, M.; McCullough, A.K.; Lloyd, R.S. Inhibition of DNA glycosylases via small molecule purine analogs. PLoS ONE 2013, 8, e81667. [Google Scholar] [CrossRef] [Green Version]
- Tahara, Y.K.; Auld, D.; Ji, D.; Beharry, A.A.; Kietrys, A.M.; Wilson, D.L.; Jimenez, M.; King, D.; Nguyen, Z.; Kool, E.T. Potent and Selective Inhibitors of 8-Oxoguanine DNA Glycosylase. J. Am. Chem. Soc. 2018, 140, 2105–2114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coste, F.; Ober, M.; Le Bihan, Y.V.; Izquierdo, M.A.; Hervouet, N.; Mueller, H.; Carell, T.; Castaing, B. Bacterial base excision repair enzyme Fpg recognizes bulky N7-substituted-FapydG lesion via unproductive binding mode. Chem. Biol. 2008, 15, 706–717. [Google Scholar] [CrossRef]
- Le Bihan, Y.V.; Angeles Izquierdo, M.; Coste, F.; Allerm, P.; Culard, F.; Gehrke, T.H.; Essalhi, K.; Carell, T.; Castaing, B. 5-Hydroxy-5-methylhydantoin DNA lesion, a molecular trap for DNA glycosylases. Nucleic Acids Res. 2011, 39, 6277–6290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castaing, B.; Geiger, A.; Seliger, H.; Nehls, P.; Laval, J.; Zelwer, C.; Boiteux, S. Cleavage and binding of a DNA fragment containing a single 8-oxoguanine by wild type and mutant FPG proteins. Nucleic Acids Res. 1993, 21, 2899–2905. [Google Scholar] [CrossRef] [Green Version]
- O’Connor, T.R.; Graves, R.J.; de Murcia, G.; Castaing, B.; Laval, J. Fpg protein of Escherichia coli is a zinc finger protein whose cysteine residues have a structural and/or functional role. J. Biol. Chem. 1993, 268, 9063–9070. [Google Scholar] [PubMed]
- Tchou, J.; Michaels, M.L.; Miller, J.H.; Grollman, A.P. Function of the zinc finger in Escherichia coli Fpg protein. J. Biol. Chem. 1993, 268, 26738–26744. [Google Scholar]
- Doublie, S.; Bandaru, V.; Bond, J.P.; Wallace, S.S. The crystal structure of human endonuclease VIII-like 1 (NEIL1) reveals a zincless finger motif required for glycosylase activity. Proc. Natl. Acad. Sci. USA 2004, 101, 10284–10289. [Google Scholar] [CrossRef] [Green Version]
- Zhu, C.; Lu, L.; Zhang, J.; Yue, Z.; Song, J.; Zong, S.; Liu, M.; Stovicek, O.; Gao, Y.Q.; Yi, C. Tautomerization-dependent recognition and excision of oxidation damage in base-excision DNA repair. Proc. Natl. Acad. Sci. USA 2016, 113, 7792–7797. [Google Scholar] [CrossRef] [Green Version]
- Boiteux, S.; Coste, F.; Castaing, B. Repair of 8-oxo-7,8-dihydroguanine in prokaryotic and eukaryotic cells: Properties and biological roles of the Fpg and OGG1 DNA N-glycosylases. Free Radic. Biol. Med. 2017, 107, 179–201. [Google Scholar] [CrossRef]
- Speina, E.; Ciesla, J.M.; Graziewicz, M.A.; Laval, J.; Kazimierczuk, Z.; Tudek, B. Inhibition of DNA repair glycosylases by base analogs and tryptophan pyrolysate, Trp-P-1. Acta Biochim. Pol. 2005, 52, 167–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boiteux, S.; O’Connor, T.R.; Lederer, F.; Gouyette, A.; Laval, J. Homogeneous Escherichia coli FPG protein. A DNA glycosylase which excises imidazole ring-opened purines and nicks DNA at apurinic/apyrimidinic sites. J. Biol. Chem. 1990, 265, 3916–3922. [Google Scholar]
- Karakaya, A.; Jaruga, P.; Bohr, V.A.; Grollman, A.P.; Dizdaroglu, M. Kinetics of excision of purine lesions from DNA by Escherichia coli Fpg protein. Nucleic Acids Res. 1997, 25, 474–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tudek, B.; Van Zeeland, A.A.; Kusmierek, J.T.; Laval, J. Activity of Escherichia coli DNA-glycosylases on DNA damaged by methylating and ethylating agents and influence of 3-substituted adenine derivatives. Mutat. Res. 1998, 407, 169–176. [Google Scholar] [CrossRef]
- Castaing, B.; Fourrey, J.L.; Hervouet, N.; Thomas, M.; Boiteux, S.; Zelwer, C. AP site structural determinants for Fpg specific recognition. Nucleic Acids Res. 1999, 27, 608–615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira de Jesus, K.; Serre, L.; Zelwerm, C.; Castaing, B. Structural insights into abasic site for Fpg specific binding and catalysis: Comparative high-resolution crystallographic studies of Fpg bound to various models of abasic site analogs-containing DNA. Nucleic Acids Res. 2005, 33, 5936–5944. [Google Scholar] [CrossRef] [Green Version]
- Serre, L.; Pereira de Jesus, K.; Boiteux, S.; Zelwer, C.; Castaing, B. Crystal structure of the Lactococcus lactis formamidopyrimidine-DNA glycosylase bound to an abasic site analog-containing DNA. EMBO J. 2002, 21, 2854–2865. [Google Scholar] [CrossRef] [Green Version]
- Castaing, B.; Boiteux, S.; Zelwer, C. DNA containing a chemically reduced apurinic site is a high affinity ligand for the E. coli formamidopyrimidine-DNA glycosylase. Nucleic Acids Res. 1992, 20, 389–394. [Google Scholar] [CrossRef]
- Sadeghian, K.; Flaig, D.; Blank, I.D.; Schneider, S.; Strasser, R.; Stathis, D.; Winnacker, M.; Carell, T.; Ochsenfeld, C. Ribose-protonated DNA base excision repair: A combined theoretical and experimental study. Angew. Chem. 2014, 53, 10044–10048. [Google Scholar] [CrossRef]
- Duclos, S.; Aller, P.; Jaruga, P.; Dizdaroglu, M.; Wallace, S.S.; Doublie, S. Structural and biochemical studies of a plant formamidopyrimidine-DNA glycosylase reveal why eukaryotic Fpg glycosylases do not excise 8-oxoguanine. DNA Repair 2012, 11, 714–725. [Google Scholar] [CrossRef] [Green Version]
- Imamura, K.; Averill, A.; Wallace, S.S.; Doublie, S. Structural characterization of viral ortholog of human DNA glycosylase NEIL1 bound to thymine glycol or 5-hydroxyuracil-containing DNA. J. Biol. Chem. 2012, 287, 4288–4298. [Google Scholar] [CrossRef] [Green Version]
- Rogacheva, M.; Ishchenko, A.; Saparbaev, M.; Kuznetsova, S.; Ogryzko, V. High resolution characterization of formamidopyrimidine-DNA glycosylase interaction with its substrate by chemical cross-linking and mass spectrometry using substrate analogs. J. Biol. Chem. 2006, 281, 32353–32365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saparbaev, M.; Sidorkina, O.M.; Jurado, J.; Privezentzev, C.V.; Greenberg, M.M.; Laval, J. Repair of oxidized purines and damaged pyrimidines by E. coli Fpg protein: Different roles of proline 2 and lysine 57 residues. Environ. Mol. Mutagenesis 2002, 39, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Duwat, P.; de Oliveira, R.; Ehrlich, S.D.; Boiteux, S. Repair of oxidative DNA damage in gram-positive bacteria: The Lactococcus lactis Fpg protein. Microbiology 1995, 141, 411–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira de Jesus, K.; Serre, L.; Hervouet, N.; Bouckson-Castaing, V.; Zelwer, C.; Castaing, B. Crystallization and preliminary X-ray crystallographic studies of a complex between the Lactococcus lactis Fpg DNA-repair enzyme and an abasic site containing DNA. Acta Cryst. D Biol. Crystallogr. 2002, 58, 679–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Meur, R.; Culard, F.; Nadan, V.; Goffinont, S.; Coste, F.; Guerin, M.; Loth, K.; Landon, C.; Castaing, B. The nucleoid-associated protein HU enhances 8-oxoguanine base excision by the formamidopyrimidine-DNA glycosylase. Biochem. J. 2015, 471, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Ryckaert, J.P.; Ciccotti, G.; Berendsen, H.J.C. Numerical integration of the cartesian equations of motion of a system with constraints: Molecular dynamics of n-alkanes. J. Comput. Phys. 1977, 23, 327–341. [Google Scholar] [CrossRef] [Green Version]
- Essmann, U.; Perera, L.; Berkowitz, M.L.; Darden, T.; Lee, H.; Pedersen, L.G. A smooth particle mesh Ewald method. J. Chem. Phys. 1995, 103, 8577–8593. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Cieplak, P.; Kollman, P.A. How, does a restrained electrostatic potential (RESP) model perform in calculating conformational energies of organic and biological molecules. J. Comput. Chem. 2000, 21, 1049–1074. [Google Scholar] [CrossRef]
- Cornell, W.D.; Cieplak, P.; Bayly, C.I.; Gould, I.R.; Merz, K.M.; Ferguson, D.M.; Spellmeyer, D.C.; Fox, T.; Caldwell, J.W.; Kollman, P.A. A second Generation Force Field for the Simulation of Proteins. J. Am. Chem. Soc. 1995, 117, 5179–5197. [Google Scholar] [CrossRef] [Green Version]
- Kabsch, W. Xds. Acta Crystallogr. D Biol. Crystallogr. 2010, 66, 125–132. [Google Scholar] [CrossRef] [Green Version]
- Winn, M.D.; Ballard, C.C.; Cowtan, K.D.; Dodson, E.J.; Emsley, P.; Evans, P.R.; Keegan, R.M.; Krissinel, E.B.; Leslie, A.G.; McCoy, A.; et al. Overview of the CCP4 suite and current developments. Acta Crystallogr. D Biol. Crystallogr. 2011, 67, 235–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McNicholas, S.; Potterton, E.; Wilson, K.S.; Noble, M.E. Presenting your structures: The CCP4mg molecular-graphics software. Acta Crystallogr. D Biol. Crystallogr. 2011, 67, 386–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goula, A.V.; Merienne, K. Abnormal base excision repair at trinucleotide repeats associated with diseases: A tissue-selective mechanism. Genes (Basel) 2013, 4, 375–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, G.; Yuan, K.; Yan, C.; Fox, J., 3rd; Gaid, M.; Breitwieser, W.; Bansal, A.K.; Zeng, H.; Gao, H.; Wu, M. 8-Oxoguanine-DNA glycosylase 1 deficiency modifies allergic airway inflammation by regulating STAT6 and IL-4 in cells and in mice. Free Radic. Biol. Med. 2012, 52, 392–401. [Google Scholar] [CrossRef] [Green Version]
- Touati, E.; Michel, V.; Thiberge, J.M.; Ave, P.; Huerre, M.; Bourgade, F.; Klungland, A.; Labigne, A. Deficiency in OGG1 protects against inflammation and mutagenic effects associated with H. pylori infection in mouse. Helicobacter 2006, 11, 494–505. [Google Scholar] [CrossRef]
- Visnes, T.; Cazares-Korner, A.; Hao, W.; Wallner, O.; Masuyer, G.; Loseva, O.; Mortusewicz, O.; Wiita, E.; Sarno, A.; Manoilov, A.; et al. Small-molecule inhibitor of OGG1 suppresses proinflammatory gene expression and inflammation. Science 2018, 362, 834–839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gavande, N.S.; VanderVere-Carozza, P.S.; Hinshaw, H.D.; Jalal, S.I.; Sears, C.R.; Pawelczak, K.S. DNA repair targeted therapy: The past or future of cancer treatment? Pharmacol. Ther. 2016, 160, 65–83. [Google Scholar] [CrossRef]
- Helleday, T. Cancer phenotypic lethality, exemplified by the non-essential MTH1 enzyme being required for cancer survival. Ann. Oncol. 2014, 25, 1253–1255. [Google Scholar] [CrossRef]
- Visnes, T.; Grube, M.; Hanna, B.M.F.; Benitez-Buelga, C.; Cazares-Korner, A.; Helleday, T. Targeting BER enzymes in cancer therapy. DNA Repair 2018, 71, 118–126. [Google Scholar] [CrossRef]
- Grin, I.R.; Rieger, R.A.; Zharkov, D.O. Inactivation of NEIL2 DNA glycosylase by pyridoxal phosphate reveals a loop important for substrate binding. Biochem. Biophys. Res. Commun. 2010, 394, 100–105. [Google Scholar] [CrossRef] [Green Version]
- Michel, M.; Visnes, T.; Homan, E.J.; Seashore-Ludlow, B.; Hedenstrom, M.; Wiita, E.; Vallin, K.; Paulin, C.B.J.; Zhang, J.; Wallner, O.; et al. Computational and Experimental Druggability Assessment of Human DNA Glycosylases. ACS Omega 2019, 4, 11642–11656. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Bao, B.B.; Song, G.Q.; Chen, C.; Zhang, X.M.; Lu, W.; Wang, Z.; Cai, Y.; Li, S.; Fu, S.; et al. Discovery of unsymmetrical aromatic disulfides as novel inhibitors of SARS-CoV main protease: Chemical synthesis, biological evaluation, molecular docking and 3D-QSAR study. Eur. J. Med. Chem. 2017, 137, 450–461. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.S.; Wang, W.M.; Lu, W.; Niu, C.W.; Li, Y.H.; Li, Z.M.; Wang, J.G. Synthesis and biological evaluation of nonsymmetrical aromatic disulfides as novel inhibitors of acetohydroxyacid synthase. Bioorganic Med. Chem. Lett. 2013, 23, 3723–3727. [Google Scholar] [CrossRef] [PubMed]
- Turos, E.; Revell, K.D.; Ramaraju, P.; Gergeres, D.A.; Greenhalgh, K.; Young, A.; Sathyanarayan, N.; Dickey, S.; Lim, D.; Alhamadsheh, M.M.; et al. Unsymmetric aryl-alkyl disulfide growth inhibitors of methicillin-resistant Staphylococcus aureus and Bacillus anthracis. Bioorg. Med. Chem. 2008, 16, 6501–6508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rabbani, G.; Ahn, S.N. Structure, enzymatic activities, glycation and therapeutic potential of human serum albumin: A natural cargo. Int. J. Biol. Macromol. 2019, 123, 979–990. [Google Scholar] [CrossRef] [PubMed]
- Rice, W.G.; Baker, D.C.; Schaeffer, C.A.; Graham, L.; Bu, M.; Terpening, S.; Clanton, D.; Schultz, R.; Bader, J.P.; Buckheit, R.W., Jr.; et al. Inhibition of multiple phases of human immunodeficiency virus type 1 replication by a dithiane compound that attacks the conserved zinc fingers of retroviral nucleocapsid proteins. Antimicrob. Agents Chemother. 1997, 41, 419–426. [Google Scholar] [CrossRef] [Green Version]
- Hong, S.; Shin, Y.; Jung, M.; Ha, M.W.; Park, Y.; Lee, Y.J.; Shin, J.; Oh, K.B.; Lee, S.K.; Park, H.G. Efficient synthesis and biological activity of Psammaplin A and its analogs as antitumor agents. Eur. J. Med. Chem. 2015, 96, 218–230. [Google Scholar] [CrossRef]
- Boerzel, H.; Koeckert, M.; Bu, W.; Spingler, B.; Lippard, S.J. Zinc-bound thiolate-disulfide exchange: A strategy for inhibiting metallo-beta-lactamases. Inorg. Chem. 2003, 42, 1604–1615. [Google Scholar] [CrossRef] [Green Version]
- Garg, D.; Torbett, B.E. Advances in targeting nucleocapsid-nucleic acid interactions in HIV-1 therapy. Virus Res. 2014, 193, 135–143. [Google Scholar] [CrossRef] [Green Version]
- Otto, S. Dynamic molecular networks: From synthetic receptors to self-replicators. Acc. Chem. Res. 2012, 45, 2200–2210. [Google Scholar] [CrossRef] [PubMed]
- Mondal, M.; Hirsch, A.K. Dynamic combinatorial chemistry: A tool to facilitate the identification of inhibitors for protein targets. Chem. Soc. Rev. 2015, 44, 2455–2488. [Google Scholar] [CrossRef] [PubMed] [Green Version]











| Compound | IC50appA (µM) (Fpg/Nei enzyme catalysis) | IC50appB (µM) (Fpg DNA binding) * | ||||
|---|---|---|---|---|---|---|
| LlFpg | hNEIL1 | mvNEI1 | C1 | C2 | ||
| 2TX * |  | 48 ± 4 | >500 | >500 | 113.3 ± 17 (453) | NA (9.7) |
| TX13 * |  | 7.5 ± 1.0 | >250 | >500 | 6.1 ± 0.5 (98) | NA (1.3) |
| TX14 * |  | 28 ± 7 | >500 | >500 | 25.1±2.4 (100) | NA (2.8) |
| TX16 ** |  | 41 ± 7 | 21 ± 1 | 14 ± 1 | ND | NA |
| TX19 ** |  | 15 ± 1 | 19 ± 1 | 124 ± 4 | 17.1 ± 2.4 (68) | NA (2) |
| TX27 * |  | 20 ± 2 | 160 ± 16 | 277 ± 18 | 44.19 ± 8.0 (177) | NA (4) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rieux, C.; Goffinont, S.; Coste, F.; Tber, Z.; Cros, J.; Roy, V.; Guérin, M.; Gaudon, V.; Bourg, S.; Biela, A.; et al. Thiopurine Derivative-Induced Fpg/Nei DNA Glycosylase Inhibition: Structural, Dynamic and Functional Insights. Int. J. Mol. Sci. 2020, 21, 2058. https://doi.org/10.3390/ijms21062058
Rieux C, Goffinont S, Coste F, Tber Z, Cros J, Roy V, Guérin M, Gaudon V, Bourg S, Biela A, et al. Thiopurine Derivative-Induced Fpg/Nei DNA Glycosylase Inhibition: Structural, Dynamic and Functional Insights. International Journal of Molecular Sciences. 2020; 21(6):2058. https://doi.org/10.3390/ijms21062058
Chicago/Turabian StyleRieux, Charlotte, Stéphane Goffinont, Franck Coste, Zahira Tber, Julien Cros, Vincent Roy, Martine Guérin, Virginie Gaudon, Stéphane Bourg, Artur Biela, and et al. 2020. "Thiopurine Derivative-Induced Fpg/Nei DNA Glycosylase Inhibition: Structural, Dynamic and Functional Insights" International Journal of Molecular Sciences 21, no. 6: 2058. https://doi.org/10.3390/ijms21062058
APA StyleRieux, C., Goffinont, S., Coste, F., Tber, Z., Cros, J., Roy, V., Guérin, M., Gaudon, V., Bourg, S., Biela, A., Aucagne, V., Agrofoglio, L., Garnier, N., & Castaing, B. (2020). Thiopurine Derivative-Induced Fpg/Nei DNA Glycosylase Inhibition: Structural, Dynamic and Functional Insights. International Journal of Molecular Sciences, 21(6), 2058. https://doi.org/10.3390/ijms21062058