Early pH Changes in Musculoskeletal Tissues upon Injury—Aerobic Catabolic Pathway Activity Linked to Inter-Individual Differences in Local pH
Abstract
:1. Introduction
2. Results
2.1. Sheep Study: pH Drop upon Hypoxia Following Injury
2.2. Gene Expression Analysis in Sheep Fracture Hematoma
2.3. Rat study: pH Drop during Hematoma Maturation Following Injury
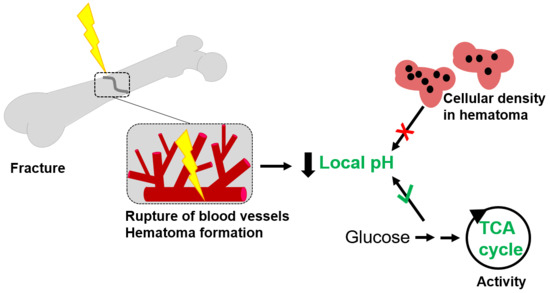
2.4. Factors Influencing the Variability in Local pH
2.4.1. The Cellularity of the Hematoma Does Not Correlate with Local pH
2.4.2. Differences in Metabolic Processes within the Hematoma Linked to Local pH
3. Discussion
4. Materials and Methods
4.1. Animal Studies
4.2. Sheep Study
4.3. Gene Expression Analysis
4.4. Rat Study
4.5. Additional Analysis of Rat Fracture Hematoma
4.5.1. Histological Analysis of Rat Fracture Hematoma
4.5.2. Metabolomics Study of Rat fracture Hematoma
4.6. Statistics
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| DPEC | Diethylpyrocarbonate water |
| GAPDH | Gylcerinaldehyde-3-phosphate dehydrogenase |
| GLUT1 | Glucose transporter 1 |
| HIF-1α | Hypoxia-inducible factor-1α |
| HMOX1 | Heme oxygenase-1 |
| H&E | Hematoxylin and eosin |
References
- Messonnier, L.; Kristensen, M.; Juel, C.; Denis, C. Importance of pH regulation and lactate/H+ transport capacity for work production during supramaximal exercise in humans. J. Appl. Physiol. 2007, 102, 1936–1944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mekhail, K.; Gunaratnam, L.; Bonicalzi, M.-E.; Lee, S. HIF activation by pH-dependent nucleolar sequestration of VHL. Nature 2004, 6, 642–647. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, B.S.; Busk, M.; Overgaard, J.; Horsman, M.; Alsner, J. Simultaneous Hypoxia and Low Extracellular pH Suppress Overall Metabolic Rate and Protein Synthesis In Vitro. PLoS ONE 2015, 10, e0134955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- King, T.C. Cell Injury, Cellular Responses to Injury, and Cell Death. In Elsevier’s Integrated Pathology; Mosby Elsevier: Philadelphia, PA, USA, 2007; pp. 1–20. [Google Scholar]
- Liberti, M.; Locasale, J.W. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef] [Green Version]
- Boidot, R.; Vegran, F.; Meulle, A.; Le Breton, A.; Dessy, C.; Sonveaux, P.; Lizard-Nacol, S.; Feron, O. Regulation of Monocarboxylate Transporter MCT1 Expression by p53 Mediates Inward and Outward Lactate Fluxes in Tumors. Cancer Res. 2011, 72, 939–948. [Google Scholar] [CrossRef] [Green Version]
- Johnson, C.H.; Ivanisevic, J.; Siuzdak, G. Metabolomics: Beyond biomarkers and towards mechanisms. Nat. Rev. Mol. Cell Boil. 2016, 17, 451–459. [Google Scholar] [CrossRef] [Green Version]
- Dargaville, T.; Farrugia, B.L.; Broadbent, J.A.; Pace, S.; Upton, Z.; Voelcker, N.H. Sensors and imaging for wound healing: A review. Biosens. Bioelectron. 2013, 41, 30–42. [Google Scholar] [CrossRef] [Green Version]
- Gisbert-Garzarán, M.; Manzano, M.; Vallet-Regi, M. pH-Responsive Mesoporous Silica and Carbon Nanoparticles for Drug Delivery. Bioengineering 2017, 4, 3. [Google Scholar] [CrossRef] [Green Version]
- Liu, N.; Yang, F.; Xiong, F.; Gu, N. The Smart Drug Delivery System and Its Clinical Potential. Theranostics 2016, 6, 1306–1323. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Ochoa, M.; Waimin, J.F.; Rahimi, R.; Ziaie, B. A pH-regulated drug delivery dermal patch for targeting infected regions in chronic wounds. Lab Chip 2019, 19, 2265–2274. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Li, J.; An, S.; Jiang, C. pH-sensitive drug-delivery systems for tumor targeting. Ther. Deliv. 2013, 4, 1499–1510. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Zhao, W.; Yu, J.; Zhao, C. Recent Development of pH-Responsive Polymers for Cancer Nanomedicine. Molecules 2018, 24, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodman, A.D.; Lemann, J.; Lennon, E.J.; Relman, A.S. Production, Excretion, and Net Balance of Fixed Acid in Patients with Renal Acidosis. J. Clin. Investig. 1965, 44, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Lemann, J.; Litzow, J.R.; Lennon, E.J. The effects of chronic acid loads in normal man: Further evidence for the participation of bone mineral in the defense against chronic metabolic acidosis. J. Clin. Investig. 1966, 45, 1608–1614. [Google Scholar] [CrossRef] [Green Version]
- Relman, A.S. The acidosis of renal disease. Am. J. Med. 1968, 44, 706–713. [Google Scholar] [CrossRef]
- Arnett, T.R.; Dempster, D.W. Perspectives: Protons and osteoclasts. J. Bone Miner. Res. 2009, 5, 1099–1103. [Google Scholar] [CrossRef]
- Arnett, T.R. Extracellular pH regulates bone cell function. J. Nutr. 2008, 138, 415S–418S. [Google Scholar] [CrossRef] [Green Version]
- Green, J.; Yamaguchi, D.T.; Kleeman, C.R.; Muallem, S. Cytosolic pH regulation in osteoblasts. Regulation of anion exchange by intracellular pH and Ca2+ ions. J. Gen. Physiol. 1990, 95, 121–145. [Google Scholar] [CrossRef]
- Kolar, P.; Schmidt-Bleek, K.; Schell, H.; Gaber, T.; Toben, D.; Schmidmaier, G.; Perka, C.; Buttgereit, F.; Duda, G.N. The Early Fracture Hematoma and Its Potential Role in Fracture Healing. Tissue Eng. Part B Rev. 2010, 16, 427–434. [Google Scholar] [CrossRef]
- Schell, H.; Duda, G.N.; Peters, A.; Tsitsilonis, S.; Johnson, K.A.; Schmidt-Bleek, K. The haematoma and its role in bone healing. J. Exp. Orthop. 2017, 4, 5. [Google Scholar] [CrossRef] [Green Version]
- Street, J.; Winter, D.; Wang, J.H.; Wakai, A.; McGuinness, A.; Redmond, H.P. Is human fracture hematoma inherently angiogenic? Clin. Orthop. Relat. Res. 2000, 378, 224–237. [Google Scholar] [CrossRef] [PubMed]
- Chakkalakal, D.A.; Mashoof, A.A.; Novák, J.; Strates, B.S.; McGuire, M.H. Mineralization and pH relationships in healing skeletal defects grafted with demineralized bone matrix. J. Biomed. Mater. Res. 1994, 28, 1439–1443. [Google Scholar] [CrossRef] [PubMed]
- Newman, R.J.; Duthie, R.B.; Francis, M.J.O. Nuclear Magnetic Resonance Studies of Fracture Repair. Clin. Orthop. Relat. Res. 1985, 198, 297–303. [Google Scholar] [CrossRef]
- Schmidt-Bleek, K.; Schell, H.; Lienau, J.; Schulz, N.; Hoff, P.; Pfaff, M.; Schmidt, G.; Martin, C.; Perka, C.; Buttgereit, F.; et al. Initial immune reaction and angiogenesis in bone healing. J. Tissue Eng. Regen. Med. 2012, 8, 120–130. [Google Scholar] [CrossRef]
- Schmidt-Bleek, K.; Schell, H.; Kolar, P.; Pfaff, M.; Perka, C.; Buttgereit, F.; Duda, G.N.; Lienau, J. Cellular composition of the initial fracture hematoma compared to a muscle hematoma: A study in sheep. J. Orthop. Res. 2009, 27, 1147–1151. [Google Scholar] [CrossRef]
- Russell, W.M.S.; Burch, R.L. The Principles of Humane Experimental Technique; Methuen & Co, Ltd.: London, UK, 1959. [Google Scholar]
- Grochot-Przeczek, A.; Kotlinowski, J.; Kozakowska, M.; Starowicz, K.; Jagodzinska, J.; Stachurska, A.; Volger, O.L.; Bukowska-Strakova, K.; Florczyk, U.; Tertil, M.; et al. Heme oxygenase-1 is required for angiogenic function of bone marrow-derived progenitor cells: Role in therapeutic revascularization. Antioxid. Redox Signal. 2014, 20, 1677–1692. [Google Scholar] [CrossRef] [Green Version]
- Higashimura, Y.; Nakajima, Y.; Yamaji, R.; Harada, N.; Shibasaki, F.; Nakano, Y.; Inui, H. Up-regulation of glyceraldehyde-3-phosphate dehydrogenase gene expression by HIF-1 activity depending on Sp1 in hypoxic breast cancer cells. Arch. Biochem. Biophys. 2011, 509, 1–8. [Google Scholar] [CrossRef]
- Yamaji, R.; Fujita, K.; Takahashi, S.; Yoneda, H.; Nagao, K.; Masuda, W.; Naito, M.; Tsuruo, T.; Miyatake, K.; Inui, H.; et al. Hypoxia up-regulates glyceraldehyde-3-phosphate dehydrogenase in mouse brain capillary endothelial cells: Involvement of Na+/Ca2+ exchanger. Biochim. Biophys. Acta 2003, 1593, 269–276. [Google Scholar] [CrossRef] [Green Version]
- Schmidt-Bleek, K.; Petersen, A.; Dienelt, A.; Schwarz, C.; Duda, G.N. Initiation and early control of tissue regeneration—bone healing as a model system for tissue regeneration. Expert Opin. Boil. Ther. 2014, 14, 247–259. [Google Scholar] [CrossRef]
- Löffler, J.; Sass, F.A.; Filter, S.; Rose, A.; Ellinghaus, A.; Duda, G.N.; Dienelt, A. Compromised Bone Healing in Aged Rats Is Associated with Impaired M2 Macrophage Function. Front. Immunol. 2019, 10, 2443. [Google Scholar] [CrossRef]
- Khacho, M.; Tarabay, M.; Patten, D.; Khacho, P.; Maclaurin, J.G.; Guadagno, J.; Bergeron, R.; Cregan, S.P.; Harper, M.-E.; Park, D.S.; et al. Acidosis overrides oxygen deprivation to maintain mitochondrial function and cell survival. Nat. Commun. 2014, 5, 3550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woo, Y.C.; Park, S.S.; Subieta, A.R.; Brennan, T. Changes in tissue pH and temperature after incision indicate acidosis may contribute to postoperative pain. Anesthesiol. 2004, 101, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Nicoll, R.; Howard, J. The acid—Ash hypothesis revisited: A reassessment of the impact of dietary acidity on bone. J. Bone Miner. Metab. 2014, 32, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Newman, R.J.; Francis, M.J.O.; Duthie, R.B. Nuclear Magnetic Resonance Studies of Experimentally Induced Delayed Fracture Union. Clin. Orthop. Relat. Res. 1987, 216, 253–261. [Google Scholar] [CrossRef]
- Wu, R.; Racker, E. Regulatory mechanisms in carbohydrate metabolism. IV. Pasteur effect and Crabtree effect in ascites tumor cells. J. Boil. Chem. 1959, 234, 1036–1041. [Google Scholar]
- Payen, V.L.; Brisson, L.; Dewhirst, M.W.; Sonveaux, P. Common Responses of Tumors and Wounds to Hypoxia. Cancer J. 2015, 21, 75–87. [Google Scholar] [CrossRef]
- Martínez-Reyes, I.; Chandel, N.S. Mitochondrial TCA cycle metabolites control physiology and disease. Nat. Commun. 2020, 11, 102–111. [Google Scholar] [CrossRef] [Green Version]
- Oyedotun, K.S.; Lemire, B.D. The Quaternary Structure of the Saccharomyces cerevisiae Succinate Dehydrogenase: Homology modeling, cofactor docking, and molecular dynamics simulation studies. J. Boil. Chem. 2003, 279, 9424–9431. [Google Scholar] [CrossRef] [Green Version]
- Chu, L.; Gao, H.; Cheng, T.; Zhang, Y.; Liu, J.; Huang, F.; Yang, C.; Shi, L.; Liu, J. A charge-adaptive nanosystem for prolonged and enhanced in vivo antibiotic delivery. Chem. Commun. 2016, 52, 6265–6268. [Google Scholar] [CrossRef]
- Einhorn, T.A.; Lane, J.M. Significant advances have been made in the way surgeons treat fractures. Clin. Orthop. Relat. Res. 1998, 335, 2–3. [Google Scholar] [CrossRef]
- Haas, N.P. Callus modulation--fiction or reality? Der Chir. 2000, 71, 987–988. [Google Scholar] [CrossRef] [PubMed]
- United States Bone and Joint Initiative. The Burden of Musculoskeletal Diseases in the United States, 3rd ed.; United States Bone and Joint Initiative: Rosemont, IL, USA, 2014. [Google Scholar]
- Ageing and Health. World Health Organization. 2018. Available online: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health (accessed on 6 January 2020).
- Manini, T.M. Development of physical disability in older adults. Curr. Aging Sci. 2011, 4, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Gruber, R.; Koch, H.; Doll, B.A.; Tegtmeier, F.; Einhorn, T.; Hollinger, J.O. Fracture healing in the elderly patient. Exp. Gerontol. 2006, 41, 1080–1093. [Google Scholar] [CrossRef] [PubMed]
- Frassetto, L.; Morris, R.C.; Sellmeyer, D.E.; Todd, K.; Sebastian, A. Diet, evolution and aging--the pathophysiologic effects of the post-agricultural inversion of the potassium-to-sodium and base-to-chloride ratios in the human diet. Eur. J. Nutr. 2001, 40, 200–213. [Google Scholar] [CrossRef]
- Frassetto, L.; Sebastian, A. How metabolic acidosis and oxidative stress alone and interacting may increase the risk of fracture in diabetic subjects. Med. Hypotheses 2012, 79, 189–192. [Google Scholar] [CrossRef]
- Gupta, A.; Atoria, C.L.; Ehdaie, B.; Shariat, S.F.; Rabbani, F.; Herr, H.W.; Bochner, B.H.; Elkin, E.B. Risk of Fracture After Radical Cystectomy and Urinary Diversion for Bladder Cancer. J. Clin. Oncol. 2014, 32, 3291–3298. [Google Scholar] [CrossRef] [Green Version]
- Kato, A.; Kido, R.; Onishi, Y.; Kurita, N.; Fukagawa, M.; Akizawa, T.; Fukuhara, S. Association of Serum Bicarbonate with Bone Fractures in Hemodialysis Patients: The Mineral and Bone Disorder Outcomes Study for Japanese CKD Stage 5D Patients (MBD-5D). Nephron Clin. Pr. 2014, 128, 79–87. [Google Scholar] [CrossRef]
- Starke, A.; Corsenca, A.; Kohler, T.; Knubben, J.; Kraenzlin, M.; Uebelhart, D.; Wüthrich, R.P.; Von Rechenberg, B.; Müller, R.; Ambühl, P.M. Correction of Metabolic Acidosis with Potassium Citrate in Renal Transplant Patients and its Effect on Bone Quality. Clin. J. Am. Soc. Nephrol. 2012, 7, 1461–1472. [Google Scholar] [CrossRef] [Green Version]
- Tabatabai, L.S.; Cummings, S.R.; Tylavsky, F.A.; Bauer, D.C.; Cauley, J.A.; Kritchevsky, S.B.; Newman, A.B.; Simonsick, E.M.; Harris, T.B.; Sebastián, A.; et al. Arterialized venous bicarbonate is associated with lower bone mineral density and an increased rate of bone loss in older men and women. J. Clin. Endocrinol. Metab. 2015, 100, 1343–1349. [Google Scholar] [CrossRef] [Green Version]
- Preininger, B.; Gerigk, H.; Bruckner, J.; Perka, C.; Schell, H.; Ellinghaus, A.; Schmidt-Bleek, K.; Duda, G. An experimental setup to evaluate innovative therapy options for the enhancement of bone healing using BMP as a benchmark—A pilot study. Eur. Cells Mater. 2012, 23, 262–272. [Google Scholar] [CrossRef]
- Lu, C.; Hansen, E.; Sapozhnikova, A.; Hu, D.; Miclau, T.; Marcucio, R.S. Effect of age on vascularization during fracture repair. J. Orthop. Res. 2008, 26, 1384–1389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hankenson, K.D.; Dishowitz, M.; Gray, C.; Schenker, M. Angiogenesis in bone regeneration. Injury 2011, 42, 556–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lähteenvuo, J.; Rosenzweig, A. Effects of aging on angiogenesis. Circ. Res. 2012, 110, 1252–1264. [Google Scholar] [CrossRef] [Green Version]
- Optical pH Sensors & Meters, version pH-18-01; PreSens Precision Sensing GmbH: Regensburg, Germany, 2018.
- Huber, C.; Klimant, I.; Krause, C.; Werner, T.; Mayr, T.; Wolfbeis, O.S. Optical sensor for seawater salinity. Anal. Bioanal. Chem. 2001, 368, 196–202. [Google Scholar] [CrossRef]
- Liebsch, G.; Klimant, I.; Krause, C.; Wolfbeis, O.S. Fluorescent imaging of pH with optical sensors using time domain dual lifetime referencing. Anal. Chem. 2001, 73, 4354–4363. [Google Scholar] [CrossRef] [PubMed]
- Beck-Broichsitter, B.E.; Christofzik, D.; Daschner, F.; Knöchel, R.; Smeets, R.; Warnke, P.; Wiltfang, J.; Becker, S.T. Endocultivation: Metabolism During Heterotopic OsteoinductionIn Vivo—Monitoring with Fiber Optic Detection Devices. Tissue Eng. Part C Methods 2012, 18, 740–746. [Google Scholar] [CrossRef]
- Jonitz, A.; Lochner, K.; Lindner, T.; Hansmann, D.; Marrot, A.; Bader, R. Oxygen consumption, acidification and migration capacity of human primary osteoblasts within a three-dimensional tantalum scaffold. J. Mater. Sci. Mater. Electron. 2011, 22, 2089–2095. [Google Scholar] [CrossRef]
- Jirkof, P.; Durst, M.; Klopfleisch, R.; Palme, R.; Thöne-Reineke, C.; Buttgereit, F.; Schmidt-Bleek, K.; Lang, A. Administration of Tramadol or Buprenorphine via the drinking water for post-operative analgesia in a mouse-osteotomy model. Sci. Rep. 2019, 9, 10749. [Google Scholar] [CrossRef] [Green Version]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Giavalisco, P.; Köhl, K.; Hummel, J.; Seiwert, B.; Willmitzer, L. 13C Isotope-Labeled Metabolomes Allowing for Improved Compound Annotation and Relative Quantification in Liquid Chromatography-Mass Spectrometry-based Metabolomic Research. Anal. Chem. 2009, 81, 6546–6551. [Google Scholar] [CrossRef]





| ∆ pH (Fracture Hematoma - Muscle Trauma) | ||||
|---|---|---|---|---|
| Time Point | 4 h | 10 h | 24 h | 48 h |
| Individual ∆ pH per animal and time point | 0.53 | −0.13 | −0.33 | −0.70 |
| 0.92 | −0.28 | −0.16 | −0.21 | |
| −0.58 | 0.13 | −0.71 | −0.51 | |
| −0.31 | 0.20 | −0.66 | 0.01 | |
| −0.78 | 0.38 | |||
| −0.71 | ||||
| Time Post Osteotomy | 10 h | 24 h | |||
|---|---|---|---|---|---|
| Local pH | 6.64 | 7.05 | 6.28 | 6.66 | 7.22 |
| D-glucose 6-phosphate/D-glucose | 0.001 | 0.003 | 0.171 | 0.004 | 0.017 |
| Pyruvic acid/D-glucose | 0.158 | 0.236 | 0.584 | 0.685 | 0.247 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berkmann, J.C.; Herrera Martin, A.X.; Ellinghaus, A.; Schlundt, C.; Schell, H.; Lippens, E.; Duda, G.N.; Tsitsilonis, S.; Schmidt-Bleek, K. Early pH Changes in Musculoskeletal Tissues upon Injury—Aerobic Catabolic Pathway Activity Linked to Inter-Individual Differences in Local pH. Int. J. Mol. Sci. 2020, 21, 2513. https://doi.org/10.3390/ijms21072513
Berkmann JC, Herrera Martin AX, Ellinghaus A, Schlundt C, Schell H, Lippens E, Duda GN, Tsitsilonis S, Schmidt-Bleek K. Early pH Changes in Musculoskeletal Tissues upon Injury—Aerobic Catabolic Pathway Activity Linked to Inter-Individual Differences in Local pH. International Journal of Molecular Sciences. 2020; 21(7):2513. https://doi.org/10.3390/ijms21072513
Chicago/Turabian StyleBerkmann, Julia C., Aaron X. Herrera Martin, Agnes Ellinghaus, Claudia Schlundt, Hanna Schell, Evi Lippens, Georg N. Duda, Serafeim Tsitsilonis, and Katharina Schmidt-Bleek. 2020. "Early pH Changes in Musculoskeletal Tissues upon Injury—Aerobic Catabolic Pathway Activity Linked to Inter-Individual Differences in Local pH" International Journal of Molecular Sciences 21, no. 7: 2513. https://doi.org/10.3390/ijms21072513
APA StyleBerkmann, J. C., Herrera Martin, A. X., Ellinghaus, A., Schlundt, C., Schell, H., Lippens, E., Duda, G. N., Tsitsilonis, S., & Schmidt-Bleek, K. (2020). Early pH Changes in Musculoskeletal Tissues upon Injury—Aerobic Catabolic Pathway Activity Linked to Inter-Individual Differences in Local pH. International Journal of Molecular Sciences, 21(7), 2513. https://doi.org/10.3390/ijms21072513