Chrysanthemi Zawadskii var. Latilobum Attenuates Obesity-Induced Skeletal Muscle Atrophy via Regulation of PRMTs in Skeletal Muscle of Mice
Abstract
:1. Introduction
2. Results
2.1. CZH Ameliorated Obesity-Induced Skeletal Muscle Accumulation of Fat and Metabolic Parameters in Obese Mice
2.2. CZH Inhibited Obesity-Induced Skeletal Muscle Atrophy in HFD-Fed Mice
2.3. CZH Increased Muscle Fiber Size and Myosin Heavy Chain (MHC) Isoform in Obese Mice
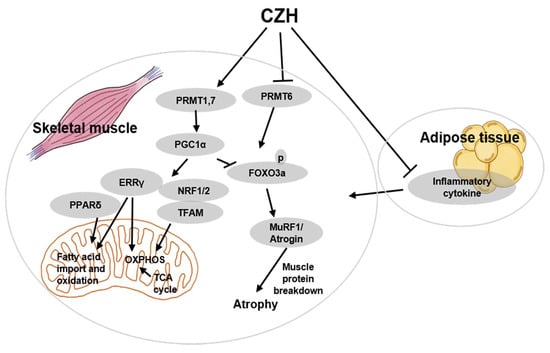
2.4. CZH Regulated Skeletal Muscle PRMTs in Obese Mice
2.5. CZH Improved Mitochondrial Dysfunction in HFD-Fed Mice
2.6. CZH, Linarin, and Luteolin Attenuated Palmitic Acid-Induced Muscle Atrophy in C2C12 Cells
3. Discussion
4. Materials and Methods
4.1. Sample Preparation
4.2. Animals
4.3. Biochemical Analysis
4.4. Treadmill and Grip Strength Tests
4.5. Histological and Immunohistochemical Analyses of Skeletal Muscle
4.6. Citrate Synthase and Mitochondrial Respiratory Complex I and II Activity
4.7. Cell Culture
4.8. Western Blot Analysis
4.9. Quantitative Real-Time PCR
4.10. mtDNA Content Quantitation
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| PPAR | peroxisome proliferator activated receptor |
| PGC1α | PPAR gamma coactivator 1α |
| FOXO3 | forkhead transcription factor3 |
| PRMT | protein arginine methyltransferase |
| CZH | Chrysanthemi zawadskii var. latilobum |
| TG | triglyceride |
| HFD | high-fat diet |
| TNFα | tumor necrosis factor-alpha |
| MCP1 | monocyte chemoattractant protein 1 |
| IL-1β | interleukin 1 beta |
| ALT | alanine aminotransferase |
| AST | aspartate aminotransferase |
| MuRF1 | muscle RING-finger protein 1 |
| Atrogin | muscle atrophy F-box |
| MHC | myosin heavy chain |
| CSA | cross-sectional area |
| NRF1 | nuclear respiratory factor 1 |
| NRF2 | nuclear factor erythroid 2-related factor 2 |
| Keap1 | kelch-like ECH-associated protein 1 |
| TFAM | mitochondrial transcription factor A |
| ERRγ | estrogen-related receptor gamma |
| mtDNA | mitochondrial DNA |
| OXPHOS | oxidative phosphorylation |
| PA | palmitic acid |
| BSA | bovine serum albumin |
| DMEM | Dulbecco’s Modified Eagle Medium |
References
- Foletta, V.C.; White, L.J.; Larsen, A.E.; Léger, B.; Russell, A.P. The role and regulation of MAFbx/atrogin-1 and MuRF1 in skeletal muscle atrophy. Pflügers Archiv Eur. J. Physiol. 2011, 461, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Nathan, J.A.; Goldberg, A.L. Muscle wasting in disease: Molecular mechanisms and promising therapies. Nat. Rev. Drug Discov. 2015, 14, 58. [Google Scholar] [CrossRef] [PubMed]
- Roy, B.; Curtis, M.E.; Fears, L.S.; Nahashon, S.N.; Fentress, H.M. Molecular mechanisms of obesity-induced osteoporosis and muscle atrophy. Front. Physiol. 2016, 7, 439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prado, C.; Wells, J.; Smith, S.; Stephan, B.; Siervo, M. Sarcopenic obesity: A critical appraisal of the current evidence. Clin. Nutr. 2012, 31, 583–601. [Google Scholar] [CrossRef]
- Kraakman, M.J.; Murphy, A.J.; Jandeleit-Dahm, K.; Kammoun, H.L. Macrophage polarization in obesity and type 2 diabetes: Weighing down our understanding of macrophage function? Front. Immunol. 2014, 5, 470. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Hickner, R.C.; Cortright, R.L.; Dohm, G.L.; Houmard, J.A. Lipid oxidation is reduced in obese human skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2000, 279, E1039–E1044. [Google Scholar] [CrossRef] [Green Version]
- Sandri, M.; Lin, J.; Handschin, C.; Yang, W.; Arany, Z.P.; Lecker, S.H.; Goldberg, A.L.; Spiegelman, B.M. PGC-1α protects skeletal muscle from atrophy by suppressing FoxO3 action and atrophy-specific gene transcription. Proc. Natl. Acad. Sci. USA 2006, 103, 16260–16265. [Google Scholar] [CrossRef] [Green Version]
- Kupr, B.; Handschin, C. Complex coordination of cell plasticity by a PGC-1α-controlled transcriptional network in skeletal muscle. Front. Physiol. 2015, 6, 325. [Google Scholar] [CrossRef] [Green Version]
- Jeong, H.-J.; Lee, H.-J.; Vuong, T.A.; Choi, K.-S.; Choi, D.; Koo, S.-H.; Cho, S.C.; Cho, H.; Kang, J.-S. Prmt7 deficiency causes reduced skeletal muscle oxidative metabolism and age-related obesity. Diabetes 2016, 65, 1868–1882. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.; Jeong, H.-J.; Kim, H.; Choi, D.; Cho, S.-C.; Seong, J.K.; Koo, S.-H.; Kang, J.-S. Skeletal muscle-specific Prmt1 deletion causes muscle atrophy via deregulation of the PRMT6-FOXO3 axis. Autophagy 2019, 15, 1069–1081. [Google Scholar] [CrossRef]
- Woo, K.-S.; Yu, J.-S.; Hwang, I.-G.; Lee, Y.-R.; Lee, C.-H.; Yoon, H.-S.; Lee, J.-S.; Jeong, H.-S. Antioxidative activity of volatile compounds in flower of Chrysanthemum indicum, C. morifolium, and C. zawadskii. J. Korean Soc. Food Sci. Nutr. 2008, 37, 805–809. [Google Scholar] [CrossRef]
- Kwon, H.S.; Ha, T.J.; Hwang, S.W.; Jin, Y.M.; Nam, S.H.; Park, K.H.; Yang, M.S. Cytotoxic flavonoids from the whole plants of Chrysanthemum zawadskii Herbich var. latilobum Kitamura. Korean Soc. Life Sci. 2006, 16, 746–749. [Google Scholar]
- He, J.; Chen, F.; Chen, S.; Lv, G.; Deng, Y.; Fang, W.; Liu, Z.; Guan, Z.; He, C. Chrysanthemum leaf epidermal surface morphology and antioxidant and defense enzyme activity in response to aphid infestation. J. Plant Physiol. 2011, 168, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Liu, H.; Yuan, Z.; Wei, D.; Ye, Y. Evaluation of antioxidant activity of chrysanthemum extracts and tea beverages by gold nanoparticles-based assay. Colloids Surf. B Biointerfaces 2012, 92, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Sung, N.-Y.; Park, Y.-Y.; Kim, Y.-E.; Cho, E.-J.; Kim, M.-H.; Ryu, G.-H.; Byun, E.-H.; Park, Y.-J. Immuno-Modulatory Activities of Polysaccharides separated from Chrysanthemum zawadskii var. latilobum in Macrophage Cells. Korean J. Food Nutr. 2016, 29, 431–437. [Google Scholar] [CrossRef] [Green Version]
- Seo, J.Y.; Lim, S.S.; Park, J.; Lim, J.-S.; Kim, H.J.; Kang, H.J.; Park, Y.; Han, J.; Kim, J.-S. Protection by Chrysanthemum zawadskii extract from liver damage of mice caused by carbon tetrachloride is maybe mediated by modulation of QR activity. Nutr. Res. Pract. 2010, 4, 93–98. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Kim, H.-K.; Lee, H.S. Hypoglycemic effect of standardized Chrysanthemum zawadskii ethanol extract in high-fat diet/streptozotocin-induced diabetic mice and rats. Food Sci. Biotechnol. 2018, 27, 1771–1779. [Google Scholar] [CrossRef]
- Park, J.A.; Jin, K.-S.; Kwon, H.J.; Kim, B.W. Antiobesity activity of Chrysanthemum zawadskii methanol extract. J. Life Sci. 2015, 25, 299–306. [Google Scholar] [CrossRef] [Green Version]
- DeFronzo, R.A.; Jacot, E.; Jequier, E.; Maeder, E.; Wahren, J.; Felber, J. The effect of insulin on the disposal of intravenous glucose: Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes 1981, 30, 1000–1007. [Google Scholar] [CrossRef]
- Sinha, I.; Sakthivel, D.; Varon, D.E. Systemic regulators of skeletal muscle regeneration in obesity. Front. Endocrinol. 2017, 8, 29. [Google Scholar] [CrossRef] [Green Version]
- Lettieri-Barbato, D.; Cannata, S.M.; Casagrande, V.; Ciriolo, M.R.; Aquilano, K. Time-controlled fasting prevents aging-like mitochondrial changes induced by persistent dietary fat overload in skeletal muscle. PLoS ONE 2018, 13, e0195912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, W.H.; Son, H.J.; Jang, Y.J.; Ahn, J.; Jung, C.H.; Ha, T.Y. Apigenin Ameliorates the Obesity-Induced Skeletal Muscle Atrophy by Attenuating Mitochondrial Dysfunction in the Muscle of Obese Mice. Mol. Nutr. Food Res. 2017, 61, 1700218. [Google Scholar] [CrossRef] [PubMed]
- Sparks, L.M.; Xie, H.; Koza, R.A.; Mynatt, R.; Hulver, M.W.; Bray, G.A.; Smith, S.R. A high-fat diet coordinately downregulates genes required for mitochondrial oxidative phosphorylation in skeletal muscle. Diabetes 2005, 54, 1926–1933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.; Hong, Y.-p.; Shin, H.J.; Lee, W. Associations of sarcopenia and sarcopenic obesity with metabolic syndrome considering both muscle mass and muscle strength. J. Prev. Med. Public Health 2016, 49, 35–44. [Google Scholar] [CrossRef]
- Stenholm, S.; Harris, T.B.; Rantanen, T.; Visser, M.; Kritchevsky, S.B.; Ferrucci, L. Sarcopenic obesity-definition, etiology and consequences. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 693–700. [Google Scholar] [CrossRef] [Green Version]
- Ceddia, R.P.; Lee, D.; Maulis, M.F.; Carboneau, B.A.; Threadgill, D.W.; Poffenberger, G.; Milne, G.; Boyd, K.L.; Powers, A.C.; McGuinness, O.P. The PGE2 EP3 receptor regulates diet-induced adiposity in male mice. Endocrinology 2016, 157, 220–232. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.-E.; Jin, B.; Li, Y.-P. TNF-α regulates myogenesis and muscle regeneration by activating p38 MAPK. Am. J. Physiol. Cell Physiol. 2007, 292, C1660–C1671. [Google Scholar] [CrossRef]
- Loell, I.; Lundberg, I. Can muscle regeneration fail in chronic inflammation: A weakness in inflammatory myopathies? J. Intern. Med. 2011, 269, 243–257. [Google Scholar] [CrossRef]
- Chabi, B.; Ljubicic, V.; Menzies, K.J.; Huang, J.H.; Saleem, A.; Hood, D.A. Mitochondrial function and apoptotic susceptibility in aging skeletal muscle. Aging Cell 2008, 7, 2–12. [Google Scholar] [CrossRef]
- Van Gurp, M.; Festjens, N.; Van Loo, G.; Saelens, X.; Vandenabeele, P. Mitochondrial intermembrane proteins in cell death. Biochem. Biophys. Res. Commun. 2003, 304, 487–497. [Google Scholar] [CrossRef]
- Marzetti, E.; Hwang, J.C.; Lees, H.A.; Wohlgemuth, S.E.; Dupont-Versteegden, E.E.; Carter, C.S.; Bernabei, R.; Leeuwenburgh, C. Mitochondrial death effectors: Relevance to sarcopenia and disuse muscle atrophy. Biochim. Biophys. Acta (BBA) Gen. Subj. 2010, 1800, 235–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balaban, R.S.; Nemoto, S.; Finkel, T. Mitochondria, oxidants, and aging. Cell 2005, 120, 483–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackson, M.J. Interactions between reactive oxygen species generated by contractile activity and aging in skeletal muscle? Antioxid. Redox Signal. 2013, 19, 804–812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ritov, V.B.; Menshikova, E.V.; He, J.; Ferrell, R.E.; Goodpaster, B.H.; Kelley, D.E. Deficiency of subsarcolemmal mitochondria in obesity and type 2 diabetes. Diabetes 2005, 54, 8–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelley, D.E.; He, J.; Menshikova, E.V.; Ritov, V.B. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes 2002, 51, 2944–2950. [Google Scholar] [CrossRef] [Green Version]
- Gleyzer, N.; Vercauteren, K.; Scarpulla, R.C. Control of mitochondrial transcription specificity factors (TFB1M and TFB2M) by nuclear respiratory factors (NRF-1 and NRF-2) and PGC-1 family coactivators. Mol. Cell. Biol. 2005, 25, 1354–1366. [Google Scholar] [CrossRef] [Green Version]
- Ventura-Clapier, R.; Garnier, A.; Veksler, V. Transcriptional control of mitochondrial biogenesis: The central role of PGC-1α. Cardiovasc. Res. 2008, 79, 208–217. [Google Scholar] [CrossRef] [Green Version]
- Virbasius, J.V.; Scarpulla, R.C. Activation of the human mitochondrial transcription factor A gene by nuclear respiratory factors: A potential regulatory link between nuclear and mitochondrial gene expression in organelle biogenesis. Proc. Natl. Acad. Sci. USA 1994, 91, 1309–1313. [Google Scholar] [CrossRef] [Green Version]
- Kaufman, B.A.; Durisic, N.; Mativetsky, J.M.; Costantino, S.; Hancock, M.A.; Grutter, P.; Shoubridge, E.A. The mitochondrial transcription factor TFAM coordinates the assembly of multiple DNA molecules into nucleoid-like structures. Mol. Biol. Cell 2007, 18, 3225–3236. [Google Scholar] [CrossRef]
- Dufour, C.R.; Wilson, B.J.; Huss, J.M.; Kelly, D.P.; Alaynick, W.A.; Downes, M.; Evans, R.M.; Blanchette, M.; Giguere, V. Genome-wide orchestration of cardiac functions by the orphan nuclear receptors ERRα and γ. Cell Metab. 2007, 5, 345–356. [Google Scholar] [CrossRef] [Green Version]
- Fan, W.; Evans, R. PPARs and ERRs: Molecular mediators of mitochondrial metabolism. Curr. Opin. Cell Biol. 2015, 33, 49–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leone, T.C.; Lehman, J.J.; Finck, B.N.; Schaeffer, P.J.; Wende, A.R.; Boudina, S.; Courtois, M.; Wozniak, D.F.; Sambandam, N.; Bernal-Mizrachi, C. PGC-1α deficiency causes multi-system energy metabolic derangements: Muscle dysfunction, abnormal weight control and hepatic steatosis. PLoS Biol. 2005, 3, e101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Exeter, D.; Connell, D.A. Skeletal muscle: Functional anatomy and pathophysiology. Semin. Musculoskelet. Radiol. 2010, 14, 097–105. [Google Scholar] [CrossRef] [PubMed]
- Bedford, M.T.; Clarke, S.G. Protein arginine methylation in mammals: Who, what, and why. Mol. Cell 2009, 33, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanc, R.S.; Richard, S. Arginine methylation: The coming of age. Mol. Cell 2017, 65, 8–24. [Google Scholar] [CrossRef] [Green Version]
- Vanlieshout, T.L.; Stouth, D.W.; Tajik, T.; Ljubicic, V. Exercise-induced Protein Arginine Methyltransferase Expression in Skeletal Muscle. Med. Sci. Sports Exerc. 2018, 50, 447–457. [Google Scholar] [CrossRef]
- Lee, Y. Studies on the constituents of Chrysanthemum sibiricum Fischer. Korean J. Pharm. Soc. 1967, 11, 7–16. [Google Scholar]
- Han, S.; Sung, K.-h.; Yim, D.; Lee, S.; Lee, C.-k.; Ha, N.-j.; Kim, K. The effect of linarin on lps-lnduced cytokine production and nitric oxide inhibition in murine macrophages cell line RAW264. 7. Arch. Pharmacal Res. 2002, 25, 170–177. [Google Scholar] [CrossRef]
- Chen, T.; Li, B.; Xu, Y.; Meng, S.; Wang, Y.; Jiang, Y. Luteolin reduces cancer-induced skeletal and cardiac muscle atrophy in a Lewis lung cancer mouse model. Oncol. Rep. 2018, 40, 1129–1137. [Google Scholar] [CrossRef] [Green Version]
- Shiota, C.; Abe, T.; Kawai, N.; Ohno, A.; Teshima-Kondo, S.; Mori, H.; Terao, J.; TANAkA, E.; NIkAWA, T. Flavones inhibit LPS-induced atrogin-1/MAFbx expression in mouse C2C12 skeletal myotubes. J. Nutr. Sci. Vitaminol. 2015, 61, 188–194. [Google Scholar] [CrossRef] [Green Version]
- HEDYA, S.; HAWILA, N.; ABDIN, A. Luteolin Attenuates Dexamethasone-Induced Skeletal Muscle Atrophy in Male Albino Rats. Med J. Cairo Univ. 2019, 87, 3365–3374. [Google Scholar]
- Kim, Y.-J.; Kim, S.-E.; Lee, H.S.; Hong, S.-Y.; Kim, S.-E.; Kim, Y.J.; Lee, J.H.; Park, S.J.; Kim, J.H.; Park, Y.-J. Comparison of linarin content and biological activity in ethanol extraction of Chrysanthemum zawadskii. J. Korean Soc. Food Sci. Nutr. 2016, 45, 1414–1421. [Google Scholar] [CrossRef]
- Hyun, M.-R.; Lee, Y.-S.; Park, Y.-H. Antioxidative activity and flavonoid content of Chrysanthemum zawadskii flowers. Hortic. Sci. Technol. 2011, 29, 68–73. [Google Scholar]
- Huynh, F.K.; Green, M.F.; Koves, T.R.; Hirschey, M.D. Measurement of fatty acid oxidation rates in animal tissues and cell lines. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2014; Volume 542, pp. 391–405. [Google Scholar]
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1α. Cell 2006, 127, 1109–1122. [Google Scholar] [CrossRef]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoo, A.; Jang, Y.J.; Ahn, J.; Jung, C.H.; Seo, H.D.; Ha, T.Y. Chrysanthemi Zawadskii var. Latilobum Attenuates Obesity-Induced Skeletal Muscle Atrophy via Regulation of PRMTs in Skeletal Muscle of Mice. Int. J. Mol. Sci. 2020, 21, 2811. https://doi.org/10.3390/ijms21082811
Yoo A, Jang YJ, Ahn J, Jung CH, Seo HD, Ha TY. Chrysanthemi Zawadskii var. Latilobum Attenuates Obesity-Induced Skeletal Muscle Atrophy via Regulation of PRMTs in Skeletal Muscle of Mice. International Journal of Molecular Sciences. 2020; 21(8):2811. https://doi.org/10.3390/ijms21082811
Chicago/Turabian StyleYoo, Ahyoung, Young Jin Jang, Jiyun Ahn, Chang Hwa Jung, Hyo Deok Seo, and Tae Youl Ha. 2020. "Chrysanthemi Zawadskii var. Latilobum Attenuates Obesity-Induced Skeletal Muscle Atrophy via Regulation of PRMTs in Skeletal Muscle of Mice" International Journal of Molecular Sciences 21, no. 8: 2811. https://doi.org/10.3390/ijms21082811
APA StyleYoo, A., Jang, Y. J., Ahn, J., Jung, C. H., Seo, H. D., & Ha, T. Y. (2020). Chrysanthemi Zawadskii var. Latilobum Attenuates Obesity-Induced Skeletal Muscle Atrophy via Regulation of PRMTs in Skeletal Muscle of Mice. International Journal of Molecular Sciences, 21(8), 2811. https://doi.org/10.3390/ijms21082811