Metabolite Profiling of Manilkara zapota L. Leaves by High-Resolution Mass Spectrometry Coupled with ESI and APCI and In Vitro Antioxidant Activity, α-Glucosidase, and Elastase Inhibition Assays
Abstract
:1. Introduction
2. Results
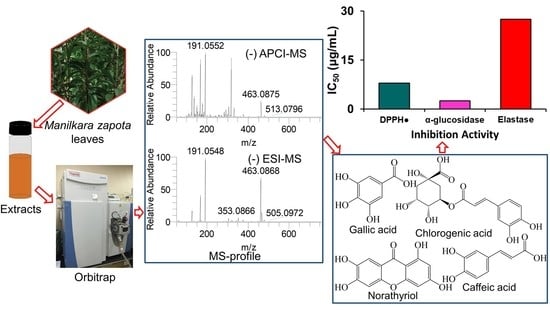
2.1. Identification of Secondary Metabolites
2.2. Antioxidant Effects of ZLE
2.3. α-Glucosidase Inhibitory Activity of ZLE
2.4. Effects of ZLE on Cell Viability and Glucose Uptake in C2C12 Myotubes
2.5. Elastase Inhibition Activity of ZLE
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Plant Materials and Extraction
4.3. Sample Preparation
4.4. Mass Spectrometry Analysis
4.5. Data Processing
4.6. Antioxidant Activity Assays
4.7. Inhibitory Assay of α-Glucosidase
4.8. Cell Culture and Cell Viability Assay
4.9. Muscle Differentiation and Glucose Uptake Assay
4.10. Elastase Inhibition Assay
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cragg, G.M.; Newman, D.J. Natural product drug discovery in the next millennium. Pharm. Biol. 2001, 39, 8–17. [Google Scholar] [PubMed] [Green Version]
- Ortega, A.M.M.; Campos, M.R.S. Bioactive compounds as therapeutic alternatives. In Bioactive Compounds: Health Benefits and Potential Applications; Chapter 13; Campos, M.R.S., Ed.; Woodhead Publishing: Sawston, UK, 2019; pp. 247–264. [Google Scholar]
- Hostettmann, K.; Wolfender, J.L.; Terreaux, C. Modern screening techniques for plant extracts. Pharm. Biol. 2001, 39, 18–32. [Google Scholar] [PubMed]
- Kaneria, M.; Chanda, S. Evaluation of antioxidant and antimicrobial properties of Manilkara zapota L. (chiku) leaves by sequential soxhlet extraction method. Asian Pac. J. Trop. Biomed. 2012, 2, S1526–S1533. [Google Scholar] [CrossRef]
- Fayek, N.M.; Monem, A.R.; Mossa, M.Y.; Meselhy, M.R.; Shazly, A.H. Chemical and biological study of Manilkara zapota (L.) Van Royen leaves (Sapotaceae) cultivated in Egypt. Pharmacogn. Res. 2012, 4, 85–91. [Google Scholar]
- Chunhakant, S.; Chaicharoenpong, C. Antityrosinase, Antioxidant, and Cytotoxic Activities of Phytochemical Constituents from Manilkara zapota L. Bark. Molecules 2019, 24, 2798. [Google Scholar] [CrossRef] [Green Version]
- Chanda, S.; Nagani, K. Antioxidant capacity of Manilkara zapota L. leaves extracts evaluated by four in vitro methods. Nat. Sci. 2010, 8, 260–266. [Google Scholar]
- Ma, J.; Luo, X.D.; Protiva, P.; Yang, H.; Ma, C.Y.; Basile, M.J.; Weinstein, I.B.; Kennelly, E.J. Bioactive novel polyphenols from the fruit of Manilkara zapota (Sapodilla). J. Nat. Prod. 2003, 66, 983–986. [Google Scholar] [CrossRef]
- Barbalho, S.M.; Bueno, P.C.D.; Delazari, D.S.; Guiguer, E.L.; Coqueiro, D.P.; Araujo, A.C.; de Souza, M.D.S.; Farinazzi-Machado, F.M.V.; Mendes, C.G.; Groppo, M. Antidiabetic and Antilipidemic Effects of Manilkara zapota. J. Med. Food 2015, 18, 385–391. [Google Scholar] [CrossRef]
- Gowda, G.A.; Djukovic, D. Overview of mass spectrometry-based metabolomics: Opportunities and challenges. Methods Mol. Biol. 2014, 1198, 3–12. [Google Scholar]
- Commisso, M.; Anesi, A.; Dal Santo, S.; Guzzo, F. Performance comparison of electrospray ionization and atmospheric pressure chemical ionization in untargeted and targeted liquid chromatography/mass spectrometry based metabolomics analysis of grapeberry metabolites. Rapid Commun. Mass Spectrom. 2017, 31, 292–300. [Google Scholar] [CrossRef]
- Sleeman, R.; Carter, J.F. Mass spectrome try|Overview. In Encyclopedia of Analytical Science, 2nd ed.; Worsfold, P., Townshend, A., Poole, C., Eds.; Elsevier: Oxford, UK, 2005; pp. 337–344. [Google Scholar]
- Lynn, K.S.; Cheng, M.L.; Chen, Y.R.; Hsu, C.; Chen, A.; Lih, T.M.; Chang, H.Y.; Huang, C.J.; Shiao, M.S.; Pan, W.H.; et al. Metabolite Identification for Mass Spectrometry-Based Metabolomics Using Multiple Types of Correlated Ion Information. Anal. Chem. 2015, 87, 2143–2151. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Lao, J.; Zhou, R.; He, W.; Qin, Y.; Zhong, C.; Xie, J.; Liu, H.; Wan, D.; Zhang, S.; et al. Simultaneous Identification and Dynamic Analysis of Saccharides during Steam Processing of Rhizomes of Polygonatum cyrtonema by HPLC⁻QTOF⁻MS/MS. Molecules 2018, 23, 2855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wishart, D.S.; Feunang, Y.D.; Marcu, A.; Guo, A.C.; Liang, K.; Vazquez-Fresno, R.; Sajed, T.; Johnson, D.; Li, C.; Karu, N.; et al. HMDB 4.0: The human metabolome database for 2018. Nucleic Acids Res. 2018, 46, D608–D617. [Google Scholar] [CrossRef] [PubMed]
- Zou, D.; Wang, J.; Zhang, B.; Xie, S.; Wang, Q.; Xu, K.; Lin, R. Analysis of Chemical Constituents in Wuzi-Yanzong-Wan by UPLC-ESI-LTQ-Orbitrap-MS. Molecules 2015, 20, 21373–21404. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.M.; Fu, Y.; Yu, W.J.; Chen, C.; Di, X.; Zhang, H.; Zhai, Y.J.; Chu, Z.Y.; Kang, T.G.; Chen, H.B. Identification of Polar Constituents in the Decoction of Juglans mandshurica and in the Medicated Egg Prepared with the Decoction by HPLC-Q-TOF MS2. Molecules 2017, 22, 1452. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Jia, Z.; Zhang, Z.; Wang, Y.; Liu, X.; Wang, L.; Lin, R. Analysis of Chemical Constituents of Melastoma dodecandrum Lour. by UPLC-ESI-Q-Exactive Focus-MS/MS. Molecules 2017, 22, 476. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.H.; Kim, B.; Kim, S.; Kim, M.S.; Kim, H.; Hwang, S.R.; Kim, K.; Lee, J.H. Characterization of metabolite profiles from the leaves of green perilla (Perilla frutescens) by ultra high performance liquid chromatography coupled with electrospray ionization quadrupole time-of-flight mass spectrometry and screening for their antioxidant properties. J. Food Drug Anal. 2017, 25, 776–788. [Google Scholar]
- Ismail, H.; Gillespie, A.L.; Calderwood, D.; Iqbal, H.; Gallagher, C.; Chevallier, O.P.; Elliott, C.T.; Pan, X.; Mirza, B.; Green, B.D. The Health Promoting Bioactivities of Lactuca sativa can be Enhanced by Genetic Modulation of Plant Secondary Metabolites. Metabolites 2019, 9, 97. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.H.; Zhang, Q.; Zhang, Y.H.; Lu, X.Y.; Fu, W.M.; He, J.Y. Chemical Analysis of Dietary Constituents in Rosa roxburghii and Rosa sterilis Fruits. Molecules 2016, 21, 1204. [Google Scholar] [CrossRef]
- Ding, M.; Jiang, Y.; Yu, X.; Zhang, D.; Li, J.; Wang, H.; Shen, J.; Gao, X.M.; Chang, Y.X. Screening of Combinatorial Quality Markers for Natural Products by Metabolomics Coupled with Chemometrics: A Case Study on Pollen Typhae. Front. Pharmacol. 2018, 9, 691. [Google Scholar] [CrossRef] [Green Version]
- Singh, D.; Siew, Y.Y.; Chong, T.I.; Yew, H.C.; Ho, S.S.; Lim, C.S.E.; Tan, W.X.; Neo, S.Y.; Koh, H.L. Identification of Phytoconstituents in Leea indica (Burm. F.) Merr. Leaves by High Performance Liquid Chromatography Micro Time-of-Flight Mass Spectrometry. Molecules 2019, 24, 714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abu-Reidah, I.M.; Ali-Shtayeh, M.S.; Jamous, R.M.; Arráez-Román, D.; Segura-Carretero, A. HPLC–DAD–ESI-MS/MS screening of bioactive components from Rhus coriaria L. (Sumac) fruits. Food Chem. 2015, 166, 179–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ancillotti, C.; Ciofi, L.; Rossini, D.; Chiuminatto, U.; Stahl-Zeng, J.; Orlandini, S.; Furlanetto, S.; Del Bubba, M. Liquid chromatographic/electrospray ionization quadrupole/time of flight tandem mass spectrometric study of polyphenolic composition of different Vaccinium berry species and their comparative evaluation. Anal. Bioanal. Chem. 2017, 409, 1347–1368. [Google Scholar] [CrossRef] [PubMed]
- Taamalli, A.; Iswaldi, I.; Arraez-Roman, D.; Segura-Carretero, A.; Fernandez-Gutierrez, A.; Zarrouk, M. UPLC-QTOF/MS for a rapid characterisation of phenolic compounds from leaves of Myrtus communis L. Phytochem. Anal. 2014, 25, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Tahri, W.; Chatti, A.; Romero-González, R.; López-Gutiérrez, N.; Frenich, A.G.; Landoulsi, A. Phenolic profiling of the aerial part of Chrysanthemum trifurcatum using ultra high performance liquid chromatography coupled to Orbitrap high resolution mass spectrometry. Anal. Methods 2016, 8, 3517–3527. [Google Scholar] [CrossRef]
- Candela, L.; Formato, M.; Crescente, G.; Piccolella, S.; Pacifico, S. Coumaroyl Flavonol Glycosides and More in Marketed Green Teas: An Intrinsic Value beyond Much-Lauded Catechins. Molecules 2020, 25, 1765. [Google Scholar] [CrossRef] [Green Version]
- Sanz, M.; de Simón, B.F.; Cadahía, E.; Esteruelas, E.; Muñoz, A.M.; Hernández, T.; Estrella, I.; Pinto, E. LC-DAD/ESI-MS/MS study of phenolic compounds in ash (Fraxinus excelsior L. and F. americana L.) heartwood. Effect of toasting intensity at cooperage. J. Mass Spectrom. 2012, 47, 905–918. [Google Scholar] [CrossRef]
- Fathoni, A.; Saepudin, E.; Cahyana, A.H.; Rahayu, D.U.C.; Haib, J. Identification of nonvolatile compounds in clove (Syzygium aromaticum) from Manado. AIP Conf. Proc. 2017, 1862, 030079. [Google Scholar]
- Guo, X.; Cheng, M.; Hu, P.; Shi, Z.; Chen, S.; Liu, H.; Shi, H.; Xu, Z.; Tian, X.; Huang, C. Absorption, Metabolism, and Pharmacokinetics Profiles of Norathyriol, an Aglycone of Mangiferin, in Rats by HPLC-MS/MS. J. Agric. Food Chem. 2018, 66, 12227–12235. [Google Scholar] [CrossRef]
- Abu-Reidah, I.M.; Arráez-Román, D.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Profiling of phenolic and other polar constituents from hydro-methanolic extract of watermelon (Citrullus lanatus) by means of accurate-mass spectrometry (HPLC–ESI–QTOF–MS). Food Res. Int. 2013, 51, 354–362. [Google Scholar] [CrossRef]
- Orlando, G.; Zengin, G.; Ferrante, C.; Ronci, M.; Recinella, L.; Senkardes, I.; Gevrenova, R.; Zheleva-Dimitrova, D.; Chiavaroli, A.; Leone, S.; et al. Comprehensive Chemical Profiling and Multidirectional Biological Investigation of Two Wild Anthemis Species (Anthemis tinctoria var. Pallida and A. cretica subsp. tenuiloba): Focus on Neuroprotective Effects. Molecules 2019, 24, 2582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Álvarez-Fernández, M.A.; Hornedo-Ortega, R.; Cerezo, A.B.; Troncoso, A.M.; García-Parrilla, M.C. Determination of Nonanthocyanin Phenolic Compounds Using High-Resolution Mass Spectrometry (UHPLC-Orbitrap-MS/MS) and Impact of Storage Conditions in a Beverage Made from Strawberry by Fermentation. J. Agric. Food Chem. 2016, 64, 1367–1376. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Narwal, S.; Kumar, V.; Prakash, O. α-glucosidase inhibitors from plants: A natural approach to treat diabetes. Pharmacogn. Rev. 2011, 5, 19–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christhudas, I.N.; Kumar, P.P.; Sunil, C.; Vajravijayan, S.; Sundaram, R.L.; Siril, S.J.; Agastian, P. In vitro studies on α-glucosidase inhibition, antioxidant and free radical scavenging activities of Hedyotis biflora L. Food Chem. 2013, 138, 1689–1695. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Wu, Z.; Wang, Z.; Zhang, H. Effect of Ethanol/Water Solvents on Phenolic Profiles and Antioxidant Properties of Beijing Propolis Extracts. Evid. Based Complement. Altern. Med. 2015, 2015, 595393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borges, A.; José, H.; Homem, V.; Simões, M. Comparison of Techniques and Solvents on the Antimicrobial and Antioxidant Potential of Extracts from Acacia dealbata and Olea europaea. Antibiotics 2020, 9, 48. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Flores, M.; Juarez-Colunga, S.; Montero-Vargas, J.M.; Lopez-Arciniega, J.A.I.; Chagolla, A.; Tiessena, A.; Winkler, R. Evaluating the physiological state of maize (Zea mays L.) plants by direct-injection electrospray mass spectrometry (DIESI-MS). Mol. Biosyst. 2012, 8, 1658–1660. [Google Scholar] [CrossRef]
- van Leerdam, J.A.; Vervoort, J.; Stroomberg, G.; de Voogt, P. Identification of Unknown Microcontaminants in Dutch River Water by Liquid Chromatography-High Resolution Mass Spectrometry and Nuclear Magnetic Resonance Spectroscopy. Environ. Sci. Technol. 2014, 48, 12791–12799. [Google Scholar] [CrossRef]
- Liotta, L.J.; James-Pederson, M. Identification of an Unknown Compound by Combined Use of IR, 1H NMR, 13C NMR, and Mass Spectrometry: A Real-Life Experience in Structure Determination. J. Chem. Educ. 2008, 85, 832. [Google Scholar] [CrossRef]
- Da Silva, L.C.; Pereira, I.; De Carvalho, T.C.; Allochio Filho, J.F.; Romão, W.; Vaz, B.G. Paper spray ionization and portable mass spectrometers: A review. Anal. Methods 2019, 11, 999–1013. [Google Scholar] [CrossRef]
- Siuzdak, G. An Introduction to Mass Spectrometry Ionization: An Excerpt from The Expanding Role of Mass Spectrometry in Biotechnology, 2nd ed.; MCC Press: San Diego, 2005. J. Assoc. Lab. Autom. 2004, 9, 50–63. [Google Scholar] [CrossRef] [Green Version]
- Das, C. Fundamentals of Contemporary Mass Spectrometry; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Farag, M.A.; Otify, A.; Porzel, A.; Michel, C.G.; Elsayed, A.; Wessjohann, L.A. Comparative metabolite profiling and fingerprinting of genus Passiflora leaves using a multiplex approach of UPLC-MS and NMR analyzed by chemometric tools. Anal. Bioanal. Chem. 2016, 408, 3125–3143. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J. Negative ion electrospray high-resolution tandem mass spectrometry of polyphenols. J. Mass Spectrom. 2016, 51, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.S.; Nair, A.G.R. Myricetin and myricetin-3-O-l-rhamnoside from the leaves of Madhuca indica and Achras sapota. Phytochemistry 1972, 11, 3090–3091. [Google Scholar] [CrossRef]
- Othman, A.; Ismail, A.; Ghani, N.A.; Adenan, I. Antioxidant capacity and phenolic content of cocoa beans. Food Chem. 2007, 100, 1523–1530. [Google Scholar] [CrossRef]
- Pientaweeratch, S.; Panapisal, V.; Tansirikongkol, A. Antioxidant, anti-collagenase and anti-elastase activities of Phyllanthus emblica, Manilkara zapota and silymarin: An in vitro comparative study for anti-aging applications. Pharm. Biol. 2016, 54, 1865–1872. [Google Scholar] [CrossRef] [Green Version]
- Pereira, D.M.; Valentao, P.; Pereira, J.A.; Andrade, P.B. Phenolics: From Chemistry to Biology. Molecules 2009, 14, 2202–2211. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Chen, D.; Shuzhi, C. Antioxidant Activity and Mechanism of Protocatechuic Acid in vitro. Funct. Foods Health Dis. 2011, 7, 232–244. [Google Scholar] [CrossRef]
- Singh, D.P.; Verma, S.; Prabha, R. Investigations on Antioxidant Potential of Phenolic Acids and Flavonoids: The Common Phytochemical Ingredients in Plants. J. Plant Biochem. Physiol. 2018, 6, 1000219. [Google Scholar] [CrossRef]
- Hsu, F.-L.; Huang, W.-J.; Wu, T.-H.; Lee, M.-H.; Chen, L.-C.; Lu, H.-J.; Hou, W.-C.; Lin, M.-H. Evaluation of antioxidant and free radical scavenging capacities of polyphenolics from pods of Caesalpinia pulcherrima. Int. J. Mol. Sci. 2012, 13, 6073–6088. [Google Scholar] [CrossRef]
- Alongi, M.; Celayeta, J.M.F.; Vriz, R.; Kinsella, G.K.; Rulikowska, A.; Anese, M. In vitro digestion nullified the differences triggered by roasting in phenolic composition and α-glucosidase inhibitory capacity of coffee. Food Chem. 2020, 128289. [Google Scholar] [CrossRef] [PubMed]
- Assefa, S.T.; Yang, E.Y.; Chae, S.Y.; Song, M.; Lee, J.; Cho, M.C.; Jang, S. Alpha Glucosidase Inhibitory Activities of Plants with Focus on Common Vegetables. Plants 2019, 9, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maitla, S. Evaluation of α-Glucosidase Inhibitory Activity of Dichloromethane and Methanol Extracts of Croton bonplandianum Baill. Trop. J. Pharm. Res. 2014, 13, 1833–1836. [Google Scholar]
- Agung, W.; Effionora, A.; Tati, N. In vitro Assay of Alpha-Glucosidase Inhibitor Activities of Three Seagrasses from Banten Bay, Indonesia. Pharmacogn. J. 2018, 10, 907–910. [Google Scholar]
- Zhang, Y.; Yang, Z.; Liu, G.; Wu, Y.; Ouyang, J. Inhibitory effect of chestnut (Castanea mollissima Blume) inner skin extract on the activity of α-amylase, α-glucosidase, dipeptidyl peptidase IV and in vitro digestibility of starches. Food Chem. 2020, 324, 126847. [Google Scholar] [CrossRef]
- Zaharudin, N.; Staerk, D.; Dragsted, L.O. Inhibition of α-glucosidase activity by selected edible seaweeds and fucoxanthin. Food Chem. 2019, 270, 481–486. [Google Scholar] [CrossRef]
- Zhang, L.; Hogan, S.; Li, J.; Sun, S.; Canning, C.; Zheng, S.J.; Zhou, K. Grape skin extract inhibits mammalian intestinal α-glucosidase activity and suppresses postprandial glycemic response in streptozocin-treated mice. Food Chem. 2011, 126, 466–471. [Google Scholar] [CrossRef]
- Lordan, S.; Smyth, T.J.; Soler-Vila, A.; Stanton, C.; Ross, R.P. The α-amylase and α-glucosidase inhibitory effects of Irish seaweed extracts. Food Chem. 2013, 141, 2170–2176. [Google Scholar] [CrossRef]
- Das, S.; De, B. In Vitro Inhibition of Key Enzymes Related to Diabetes by the Aqueous Extracts of Some Fruits of West Bengal, India. Curr. Nutr. Food Sci. 2012, 8, 19–24. [Google Scholar]
- Oboh, G.; Agunloye, O.M.; Adefegha, S.A.; Akinyemi, A.J.; Ademiluyi, A.O. Caffeic and chlorogenic acids inhibit key enzymes linked to type 2 diabetes (in vitro): A comparative study. J. Basic Clin. Physiol. Pharm. 2015, 26, 165–170. [Google Scholar] [CrossRef]
- Deng, N.; Zheng, B.; Li, T.; Liu, R.H. Assessment of the Phenolic Profiles, Hypoglycemic Activity, and Molecular Mechanism of Different Highland Barley (Hordeum vulgare L.) Varieties. Int. J. Mol. Sci. 2020, 21, 1175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subramoniam, A. Plants with Anti-Diabetes Mellitus Properties, 1st ed.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Islam, S.; Alam, M.B.; Ahmed, A.; Lee, S.; Lee, S.-H.; Kim, S. Identification of secondary metabolites in Averrhoa carambola L. bark by high-resolution mass spectrometry and evaluation for α-glucosidase, tyrosinase, elastase, and antioxidant potential. Food Chem. 2020, 332, 127377. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Liu, X.; Jiang, Z.; Geng, S.; Ma, H.; Liu, B. Interaction Mechanism of Flavonoids and α-Glucosidase: Experimental and Molecular Modelling Studies. Foods 2019, 8, 355. [Google Scholar] [CrossRef] [Green Version]
- Koehn, F.E.; Carter, G.T. The evolving role of natural products in drug discovery. Nat. Rev. Drug Discov. 2005, 4, 206–220. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Nieves, D.; Chaikof, E.L. Collagen and Elastin Biomaterials for the Fabrication of Engineered Living Tissues. ACS Biomater. Sci. Eng. 2017, 3, 694–711. [Google Scholar] [CrossRef]
- Linsenmayer, T.F. Collagen. Cell Biology of Extracellular Matrix; Springer US: New York, NY, USA, 1991; pp. 7–44. [Google Scholar]
- Mithieux, S.M.; Weiss, A.S. Elastin. Adv. Protein Chem. 2005, 70, 437–461. [Google Scholar]
- Seite, S.; Zucchi, H.; Septier, D.; Igondjo-Tchen, S.; Senni, K.; Godeau, G. Elastin changes during chronological and photo-ageing: The important role of lysozyme. J. Eur. Acad. Dermatol. Venereol. 2006, 20, 980–987. [Google Scholar] [CrossRef]
- Kwon, K.-R.; Alam, M.B.; Park, J.-H.; Kim, T.-H.; Lee, S.-H. Attenuation of UVB-Induced Photo-Aging by Polyphenolic-Rich Spatholobus Suberectus Stem Extract Via Modulation of MAPK/AP-1/MMPs Signaling in Human Keratinocytes. Nutrients 2019, 11, 1341. [Google Scholar] [CrossRef] [Green Version]
- Pathan, M.; Keerthikumar, S.; Ang, C.S.; Gangoda, L.; Quek, C.Y.; Williamson, N.A.; Mouradov, D.; Sieber, O.M.; Simpson, R.J.; Salim, A.; et al. FunRich: An open access standalone functional enrichment and interaction network analysis tool. Proteomics 2015, 15, 2597–2601. [Google Scholar] [CrossRef]
- Alam, M.B.; Ju, M.K.; Lee, S.H. DNA Protecting Activities of Nymphaea nouchali (Burm. f) Flower Extract Attenuate t-BHP-Induced Oxidative Stress Cell Death through Nrf2-Mediated Induction of Heme Oxygenase-1 Expression by Activating MAP-Kinases. Int. J. Mol. Sci. 2017, 18, 2069. [Google Scholar] [CrossRef] [Green Version]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Apak, R.; Guclu, K.; Ozyurek, M.; Karademir, S.E. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J. Agric. Food Chem. 2004, 52, 7970–7981. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.B.; Bajpai, V.K.; Ra, J.S.; Lim, J.Y.; An, H.; Shukla, S.; Quan, K.T.; Khan, I.; Huh, Y.S.; Han, Y.K.; et al. Anthraquinone-type inhibitor of alpha-glucosidase enhances glucose uptake by activating an insulin-like signaling pathway in C2C12 myotubes. Food Chem. Toxicol. 2019, 129, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Xiong, M.; Huang, Y.; Liu, Y.; Huang, M.; Song, G.; Ming, Q.; Ma, X.; Yang, J.; Deng, S.; Wen, Y.; et al. Antidiabetic Activity of Ergosterol from Pleurotus Ostreatus in KK-A(y) Mice with Spontaneous Type 2 Diabetes Mellitus. Mol. Nutr. Food Res. 2018, 62. [Google Scholar] [CrossRef] [PubMed]




| Group | Compound Name | Elemental Formula | Observed m/z | Calculated m/z | Adducts | MS/MS Fragments | CE * (eV) | Ionizations |
|---|---|---|---|---|---|---|---|---|
| Sugar | Rhamnose | C6H12O5 | 163.0598 | 163.0611 | [M–H]− | 145, 119 | 20 | ESI, APCI |
| Glucose | C6H12O6 | 179.0547 | 179.0561 | [M–H]− | 161, 143, 113, 101 | 10 | ESI, APCI | |
| 215.0316 | 215.0324 | [M+Cl]− | 179, 161, 143, 113, 101 | 10 | ESI | |||
| Sucrose | C12H22O11 | 341.1077 | 341.1083 | [M–H]− | 179, 161, 119, 101 | 10 | ESI, APCI | |
| Dicarboxylic acid | Succinic acid | C4H6O4 | 117.0179 | 117.0193 | [M–H]− | 99, 73 | 10 | APCI |
| Malic Acid | C4H6O5 | 133.0128 | 133.0133 | 115, 89, 71 | 10 | ESI, APCI | ||
| Adipic acid | C6H10O4 | 145.0492 | 145.0506 | 127, 101 | 10 | APCI | ||
| 3-Oxoadipic acid | C6H8O5 | 159.0285 | 159.0298 | 141, 115, 97 | 10 | APCI | ||
| Phenolic acids | Salicylic acid | C7H6O3 | 137.0230 | 137.0244 | [M–H]− | 93 | 20 | ESI, APCI |
| 3,4-Dihydroxybenzoic acid | C7H6O4 | 153.0179 | 153.0193 | 109 | 10 | ESI, APCI | ||
| Vanillic acid | C8H8O4 | 167.0336 | 167.0349 | 152, 123, 108 | 10 | ESI, APCI | ||
| Gallic acid | C7H6O5 | 169.0129 | 169.0142 | 125 | 10 | ESI, APCI | ||
| Caffeic acid | C9H8O4 | 179.0336 | 179.0349 | 161, 135 | 10 | ESI, APCI | ||
| Ferulic acid | C10H10O4 | 193.0591 | 193.0506 | 178, 149, 134 | 10 | ESI, APCI | ||
| Syringic acid | C9H10O5 | 197.0442 | 197.0455 | 182, 153, 125 | 10 | ESI, APCI | ||
| Chlorogenic acid | C16H18O9 | 353.0866 | 353.0878 | 191 | 10 | ESI, APCI | ||
| Flavonoids | Afzelechin | C15H14O5 | 273.0765 | 273.0768 | [M–H]− | 167 | 10 | APCI |
| Epicatechin | C15H14O6 | 289.0708 | 289.0719 | 245, 205, 179, 137 | 20 | ESI, APCI | ||
| Epigallocatechin | C15H14O7 | 305.0656 | 305.0666 | 287, 261, 219, 179 | 10 | ESI, APCI | ||
| Myricetin | C15H10O8 | 317.0296 | 317.0302 | 193, 165, 137 | 20 | APCI | ||
| Ampelopsin | C15H12O8 | 319.0447 | 319.0459 | 193, 178, 153, 125 | 10 | ESI | ||
| Laricitrin | C16H12O8 | 331.0454 | 331.0459 | 316, 178, 151 | 20 | ESI, APCI | ||
| Myricetin-3-O-rhamnoside | C21H20O12 | 463.0868 | 463.0882 | 316 | 30 | ESI, APCI | ||
| Laricitrin-3-O-rhamnoside | C22H22O12 | 477.1022 | 477.1038 | 331, 316, 287 | 20 | ESI, APCI | ||
| Prodelphinidin B | C30H26O14 | 609.1233 | 609.1249 | 441, 423, 305, 125 | 10 | ESI | ||
| Others | 2-Hydroxybenzaldehyde | C7H6O2 | 121.0280 | 121.0295 | [M–H]− | 30 | ESI, APCI | |
| Guaiacol | C7H8O2 | 123.0437 | 123.0451 | 108, 93 | 10 | APCI | ||
| Pyroglutamic acid | C5H7NO3 | 128.0386 | 128.0353 | 82 | 20 | ESI, APCI | ||
| Threonic acid | C4H8O5 | 135.0285 | 135.0298 | 117, 91, 75 | 10 | ESI, APCI | ||
| Vanillin | C8H8O3 | 151.0388 | 151.0407 | 136 | 10 | ESI, APCI | ||
| 3-Hydroxycoumarin | C9H6O3 | 161.0230 | 161.0244 | 133, 117 | 10 | ESI, APCI | ||
| Shikimic acid | C7H10O5 | 173.0442 | 173.0455 | 155, 129 | 10 | ESI, APCI | ||
| Esculetin | C9H6O4 | 177.0179 | 177.0193 | 159, 149, 133, 121 | 10 | ESI, APCI | ||
| Quinic acid | C7H12O6 | 191.0548 | 191.0561 | 173, 127, 93, 85 | 20 | ESI, APCI | ||
| Norathyriol | C13H8O6 | 259.0242 | 259.0247 | 231, 215, 187, 171 | 30 | APCI | ||
| Hydroquinone glucuronide | C12H14O8 | 285.0615 | 285.0615 | 152, 109, 108 | 10 | ESI | ||
| Leucodelphinidin | C15H14O8 | 321.0604 | 321.0616 | 303, 195, 125 | 10 | ESI | ||
| 3-Glucogallic acid | C13H16O10 | 331.0655 | 331.0670 | 287, 241, 169, 125 | 10 | ESI | ||
| 3-p-Coumaroylquinic acid | C16H18O8 | 337.0917 | 337.0928 | 191, 173,163 | 10 | ESI | ||
| 3-O-Galloylquinic acid | C14H16O10 | 343.0658 | 343.0670 | 191,173,169, 125 | 10 | ESI | ||
| Unknown | C6H6O3 | 125.0230 | 125.0244 | [M–H]− | 96 | 10 | ESI, APCI | |
| Unknown | C24H20O10 | 467.0967 | 467.0983 | 357, 303, 217 | 10 | ESI, APCI |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islam, S.; Alam, M.B.; Ann, H.-J.; Park, J.-H.; Lee, S.-H.; Kim, S. Metabolite Profiling of Manilkara zapota L. Leaves by High-Resolution Mass Spectrometry Coupled with ESI and APCI and In Vitro Antioxidant Activity, α-Glucosidase, and Elastase Inhibition Assays. Int. J. Mol. Sci. 2021, 22, 132. https://doi.org/10.3390/ijms22010132
Islam S, Alam MB, Ann H-J, Park J-H, Lee S-H, Kim S. Metabolite Profiling of Manilkara zapota L. Leaves by High-Resolution Mass Spectrometry Coupled with ESI and APCI and In Vitro Antioxidant Activity, α-Glucosidase, and Elastase Inhibition Assays. International Journal of Molecular Sciences. 2021; 22(1):132. https://doi.org/10.3390/ijms22010132
Chicago/Turabian StyleIslam, Syful, Md Badrul Alam, Hyeon-Jin Ann, Ji-Hyun Park, Sang-Han Lee, and Sunghwan Kim. 2021. "Metabolite Profiling of Manilkara zapota L. Leaves by High-Resolution Mass Spectrometry Coupled with ESI and APCI and In Vitro Antioxidant Activity, α-Glucosidase, and Elastase Inhibition Assays" International Journal of Molecular Sciences 22, no. 1: 132. https://doi.org/10.3390/ijms22010132
APA StyleIslam, S., Alam, M. B., Ann, H. -J., Park, J. -H., Lee, S. -H., & Kim, S. (2021). Metabolite Profiling of Manilkara zapota L. Leaves by High-Resolution Mass Spectrometry Coupled with ESI and APCI and In Vitro Antioxidant Activity, α-Glucosidase, and Elastase Inhibition Assays. International Journal of Molecular Sciences, 22(1), 132. https://doi.org/10.3390/ijms22010132