Involvement of cAMP-Dependent Protein Kinase in the Nucleus Accumbens in Cocaine Versus Social Interaction Reward
Abstract
:1. Introduction
2. Results
2.1. Phosphorylated PKA (pPKA) Levels were Negatively Correlated with Cocaine Preference in the NAc
2.1.1. Conditioned Place Preference for Cocaine or Social Interaction
2.1.2. PKA Expression in the NAc after Cocaine or Social Interaction Preference
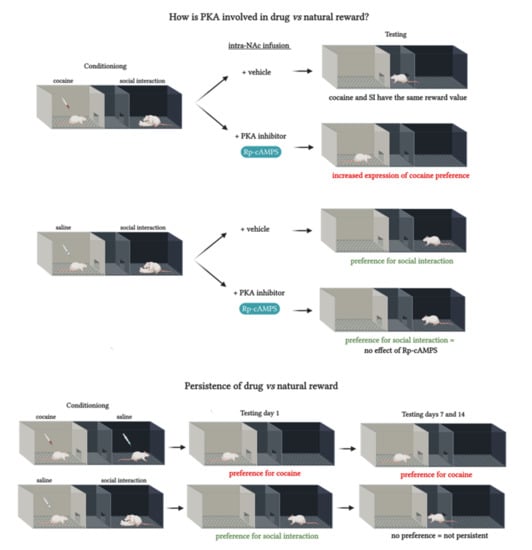
2.2. PKA Inhibition in the NAc Shifted the Preference Toward Cocaine
2.3. NAc Infusion of Rp-cAMPS Reduced Levels of DARPP-32 Phosphorylation
2.4. PKA Inhibition in the NAc did not Affect Social Interaction Reward
2.5. Persistence of Drug vs. Behavior Non-drug Conditioned Place Preference
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Place Conditioning Procedure
4.2.1. Apparatus
4.2.2. Acquisition of Place Preference
4.2.3. Concurrent CPP Paradigm
4.2.4. Persistence of Social Interaction/Cocaine CPP
4.3. Incorrect Transitions of Cephalocaudal Grooming Progression
4.4. Surgeries and Intra-NAc Infusions
4.5. Western Blot
4.6. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | analysis of variance |
| BSA | bovine serum albumin |
| CA | conditioned activity |
| cAMP | cyclic adenosine monophosphate |
| CPP | conditioned place preference |
| CREB | cAMP response element-binding protein |
| DARPP-32 | dopamine-regulated phosphoprotein-32 |
| i.p. | intraperitoneal |
| Icv | intracerebroventricular |
| NAc | nucleus accumbens |
| NFDM | non-fat dry milk |
| pCREB | phosphorylated cAMP response element-binding protein |
| pDARPP-32 | phosphorylated dopamine-regulated phosphoprotein-32 |
| PKA | protein kinase A: cAMP-dependent protein kinase |
| pPKA | phosphorylated protein kinase A |
| RIPA | radioimmunoprecipitation assay |
| RT | room temperature |
| SI | social interaction |
| TBST-T | tris-buffered saline with Tween 20 |
References
- Kelley, A.E. Memory and addiction: Shared neural circuitry and molecular mechanisms. Neuron 2004, 44, 161–179. [Google Scholar] [CrossRef] [Green Version]
- Gerdjikov, T.V.; Giles, A.C.; Swain, S.N.; Beninger, R.J. Nucleus accumbens PKA inhibition blocks acquisition but enhances expression of amphetamine-produced conditioned activity in rats. Psychopharmacology 2007, 190, 65–72. [Google Scholar] [CrossRef]
- Beninger, R.J.; Nakonechny, P.L.; Savina, I. cAMP-dependent protein kinase and reward-related learning: Intra-accumbens Rp-cAMPS blocks amphetamine-produced place conditioning in rats. Psychopharmacology 2003, 170, 23–32. [Google Scholar] [CrossRef]
- Beninger, R.J.; Gerdjikov, T. The role of signaling molecules in reward-related incentive learning. Neurotox. Res. 2004, 6, 91–103. [Google Scholar] [CrossRef]
- Cervo, L.; Mukherjee, S.; Bertaglia, A.; Samanin, R. Protein kinases A and C are involved in the mechanisms underlying consolidation of cocaine place conditioning. Brain Res. 1997, 775, 30–36. [Google Scholar] [CrossRef]
- Sutton, M.A.; McGibney, K.; Beninger, R.J. Conditioned locomotion in rats following amphetamine infusion into the nucleus accumbens: Blockade by coincident inhibition of protein kinase A. Behav. Pharmacol. 2000, 11, 365–376. [Google Scholar] [CrossRef] [Green Version]
- Miserendino, M.J.D.; Nestler, E.J. Behavioral sensitization to cocaine: Modulation by the cyclic AMP system in the nucleus accumbens. Brain Res. 1995, 674, 299–306. [Google Scholar] [CrossRef]
- Lynch, W.J.; Taylor, J.R. Persistent changes in motivation to self-administer cocaine following modulation of cyclic AMP-dependent protein kinase A (PKA) activity in the nucleus accumbens. Eur. J. Neurosci. 2005, 22, 1214–1220. [Google Scholar] [CrossRef]
- Self, D.W.; Genova, L.M.; Hope, B.T.; Barnhart, W.J.; Spencer, J.J.; Nestler, E.J. Involvement of cAMP-dependent protein kinase in the nucleus accumbens in cocaine self-administration and relapse of cocaine-seeking behavior. J. Neurosci. 1998, 18, 1848–1859. [Google Scholar] [CrossRef]
- El Rawas, R.; Saria, A. The Two Faces of Social Interaction Reward in Animal Models of Drug Dependence. Neurochem. Res. 2016, 41, 492–499. [Google Scholar] [CrossRef] [Green Version]
- EL Rawas, R.; Amaral, I.M.; Hofer, A. Social interaction reward: A resilience approach to overcome vulnerability to drugs of abuse. Eur. Neuropsychopharmacol. 2020, 37, 12–28. [Google Scholar] [CrossRef]
- Kummer, K.; Klement, S.; Eggart, V.; Mayr, M.J.; Saria, A.; Zernig, G. Conditioned place preference for social interaction in rats: Contribution of sensory components. Front. Behav. Neurosci. 2011, 5, 80. [Google Scholar] [CrossRef] [Green Version]
- Peartree, N.A.; Hood, L.E.; Thiel, K.J.; Sanabria, F.; Pentkowski, N.S.; Chandler, K.N.; Neisewander, J.L. Limited physical contact through a mesh barrier is sufficient for social reward-conditioned place preference in adolescent male rats. Physiol. Behav. 2012, 105, 749–756. [Google Scholar] [CrossRef] [Green Version]
- Fritz, M.; El Rawas, R.; Salti, A.; Klement, S.; Bardo, M.T.; Kemmler, G.; Dechant, G.; Saria, A.; Zernig, G. Reversal of cocaine-conditioned place preference and mesocorticolimbic Zif268 expression by social interaction in rats. Addict. Biol. 2011, 16, 273–284. [Google Scholar] [CrossRef]
- Bregolin, T.; Pinheiro, B.S.; El Rawas, R.; Zernig, G. Preventive strength of dyadic social interaction against reacquisition/reexpression of cocaine conditioned place preference. Front. Behav. Neurosci. 2017, 11. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro Do Couto, B.; Aguilar, M.A.; Lluch, J.; Rodríguez-Arias, M.; Miñarro, J. Social experiences affect reinstatement of cocaine-induced place preference in mice. Psychopharmacology 2009, 207, 485–498. [Google Scholar] [CrossRef]
- Lemos, C.; Salti, A.; Amaral, I.M.; Fontebasso, V.; Singewald, N.; Dechant, G.; Hofer, A.; El Rawas, R. Social interaction reward in rats has anti-stress effects. Addict. Biol. 2020, 26, e12878. [Google Scholar] [CrossRef] [Green Version]
- Venniro, M.; Zhang, M.; Caprioli, D.; Hoots, J.K.; Golden, S.A.; Heins, C.; Morales, M.; Epstein, D.H.; Shaham, Y. Volitional social interaction prevents drug addiction in rat models. Nat. Neurosci. 2018, 21, 1520–1529. [Google Scholar] [CrossRef]
- El Rawas, R.; Klement, S.; Kummer, K.K.; Fritz, M.; Dechant, G.; Saria, A.; Zernig, G. Brain regions associated with the acquisition of conditioned place preference for cocaine vs. social interaction. Front. Behav. Neurosci. 2012, 6, 63. [Google Scholar] [CrossRef] [Green Version]
- Olsen, C.M. Natural rewards, neuroplasticity, and non-drug addictions. Neuropharmacology 2011, 61, 1109–1122. [Google Scholar] [CrossRef] [Green Version]
- Been, L.E.; Hedges, V.L.; Vialou, V.; Nestler, E.J.; Meisel, R.L. ΔJunD overexpression in the nucleus accumbens prevents sexual reward in female Syrian hamsters. Genes Brain Behav. 2013, 12, 666–672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pitchers, K.K.; Vialou, V.; Nestler, E.J.; Laviolette, S.R.; Lehman, M.N.; Coolen, L.M. Natural and drug rewards act on common neural plasticity mechanisms with ΔFosB as a key mediator. J. Neurosci. 2013, 33, 3434–3442. [Google Scholar] [CrossRef] [PubMed]
- Reichel, C.M.; Wilkinson, J.L.; Bevins, R.A. Reference place conditioning procedure with cocaine: Increased sensitivity for measuring associatively motivated choice behavior in rats. Behav. Pharmacol. 2010, 21, 323–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fritz, M.; El Rawas, R.; Klement, S.; Kummer, K.; Mayr, M.J.; Eggart, V.; Salti, A.; Bardo, M.T.; Saria, A.; Zernig, G. Differential effects of accumbens core vs. shell lesions in a rat concurrent conditioned place preference paradigm for cocaine vs. social interaction. PLoS ONE 2011, 6, e26761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, L.; Grimm, J.W.; Shaham, Y.; Hope, B.T. Molecular neuroadaptations in the accumbens and ventral tegmental area during the first 90 days of forced abstinence from cocaine self-administration in rats. J. Neurochem. 2003, 85, 1604–1613. [Google Scholar] [CrossRef] [Green Version]
- Terwilliger, R.Z.; Beitner-Johnson, D.; Sevarino, K.A.; Crain, S.M.; Nestler, E.J. A general role for adaptations in G-proteins and the cyclic AMP system in mediating the chronic actions of morphine and cocaine on neuronal function. Brain Res. 1991, 548, 100–110. [Google Scholar] [CrossRef]
- Freeman, W.M.; Nader, M.A.; Nader, S.H.; Robertson, D.J.; Gioia, L.; Mitchell, S.M.; Daunais, J.B.; Porrino, L.J.; Friedman, D.P.; Vrana, K.E. Chronic cocaine-mediated changes in non-human primate nucleus accumbens gene expression. J. Neurochem. 2001, 77, 542–549. [Google Scholar] [CrossRef]
- Hope, B.T.; Crombag, H.S.; Jedynak, J.P.; Wise, R.A. Neuroadaptations of total levels of adenylate cyclase, protein kinase A, tyrosine hydroxylase, cdk5 and neurofilaments in the nucleus accumbens and ventral tegmental area do not correlate with expression of sensitized or tolerant locomotor responses to cocaine. J. Neurochem. 2005, 92, 536–545. [Google Scholar] [CrossRef]
- Unterwald, E.M.; Fillmore, J.; Kreek, M.J. Chronic repeated cocaine administration increases dopamine D1 receptor-mediated signal transduction. Eur. J. Pharmacol. 1996, 318, 31–35. [Google Scholar] [CrossRef]
- Crawford, C.A.; Choi, F.Y.; Kohutek, J.L.; Yoshida, S.T.; McDougall, S.A. Changes in PKA Activity and Gsα and Golfα Levels after Amphetamine and Cocaine-Induced Behavioral Sensitization. Synapse 2004, 51, 241–248. [Google Scholar] [CrossRef]
- Baldwin, A.E.; Sadeghian, K.; Holahan, M.R.; Kelley, A.E. Appetitive instrumental learning is impaired by inhibition of cAMP-dependent protein kinase within the nucleus accumbens. Neurobiol. Learn. Mem. 2002, 77, 44–62. [Google Scholar] [CrossRef]
- Wang, L.; Guo, T.; Guo, Y.; Xu, Y. Asiaticoside produces an antidepressant—Like effect in a chronic unpredictable mild stress model of depression in mice, involving reversion of inflammation and the PKA/pCREB/BDNF signaling pathway. Mol. Med. Rep. 2020, 22, 2364–2372. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, Y.; Mondal, A.C.; Shukla, P.K.; Rizavi, H.S.; Lyons, J. Altered protein kinase A in brain of learned helpless rats: Effects of acute and repeated stress. Biol. Psychiatry 2004, 56, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Logrip, M.L.; Koob, G.F.; Zorrilla, E.P. Role of corticotropin-releasing factor in drug addiction: Potential for pharmacological intervention. CNS Drugs 2011, 25, 271–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalueff, A.V.; Tuohimaa, P. Grooming analysis algorithm for neurobehavioural stress research. Brain Res. Protoc. 2004, 13, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Kummer, K.K.; Hofhansel, L.; Barwitz, C.M.; Schardl, A.; Prast, J.M.; Salti, A.; El Rawas, R.; Zernig, G. Differences in social interaction vs. cocaine reward in mouse vs. rat. Front. Behav. Neurosci. 2014, 8, 363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Chiara, G. Nucleus accumbens shell and core dopamine: Differential role in behavior and addiction. Behav. Brain Res. 2002, 137, 75–114. [Google Scholar] [CrossRef]
- Ikemoto, S.; Qin, M.; Liu, Z.-H. The functional divide for primary reinforcement of D-amphetamine lies between the medial and lateral ventral striatum: Is the division of the accumbens core, shell, and olfactory tubercle valid? J. Neurosci. 2005, 25, 5061–5065. [Google Scholar] [CrossRef]
- Corbit, L.H.; Fischbach, S.C.; Janak, P.H. Nucleus accumbens core and shell are differentially involved in general and outcome-specific forms of Pavlovian-instrumental transfer with alcohol and sucrose rewards. Eur. J. Neurosci. 2016, 43, 1229–1236. [Google Scholar] [CrossRef]
- Floresco, S.B.; Montes, D.R.; Tse, M.M.T.; van Holstein, M. Differential contributions of nucleus accumbens subregions to cue-guided risk/reward decision making and implementation of conditional rules. J. Neurosci. 2018, 38, 1901–1914. [Google Scholar] [CrossRef]
- Misra, K.; Pandey, S.C. The decreased cyclic-AMP dependent-protein kinase A function in the nucleus accumbens: A role in alcohol drinking but not in anxiety-like behaviors in rats. Neuropsychopharmacology 2006, 31, 1406–1419. [Google Scholar] [CrossRef] [Green Version]
- Carr, G.D.; Phillips, A.G.; Fibiger, H.C. Independence of amphetamine reward from locomotor stimulation demonstrated by conditioned place preference. Psychopharmacology 1988, 94, 221–226. [Google Scholar] [CrossRef]
- Solinas, M.; Chauvet, C.; Thiriet, N.; El Rawas, R.; Jaber, M. Reversal of cocaine addiction by environmental enrichment. Proc. Natl. Acad. Sci. USA 2008, 105, 17145–17150. [Google Scholar] [CrossRef] [Green Version]
- Miller, C.A.; Marshall, J.F. Molecular substrates for retrieval and reconsolidation of cocaine-associated contextual memory. Neuron 2005, 47, 873–884. [Google Scholar] [CrossRef] [Green Version]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amaral, I.M.; Lemos, C.; Cera, I.; Dechant, G.; Hofer, A.; El Rawas, R. Involvement of cAMP-Dependent Protein Kinase in the Nucleus Accumbens in Cocaine Versus Social Interaction Reward. Int. J. Mol. Sci. 2021, 22, 345. https://doi.org/10.3390/ijms22010345
Amaral IM, Lemos C, Cera I, Dechant G, Hofer A, El Rawas R. Involvement of cAMP-Dependent Protein Kinase in the Nucleus Accumbens in Cocaine Versus Social Interaction Reward. International Journal of Molecular Sciences. 2021; 22(1):345. https://doi.org/10.3390/ijms22010345
Chicago/Turabian StyleAmaral, Inês M., Cristina Lemos, Isabella Cera, Georg Dechant, Alex Hofer, and Rana El Rawas. 2021. "Involvement of cAMP-Dependent Protein Kinase in the Nucleus Accumbens in Cocaine Versus Social Interaction Reward" International Journal of Molecular Sciences 22, no. 1: 345. https://doi.org/10.3390/ijms22010345
APA StyleAmaral, I. M., Lemos, C., Cera, I., Dechant, G., Hofer, A., & El Rawas, R. (2021). Involvement of cAMP-Dependent Protein Kinase in the Nucleus Accumbens in Cocaine Versus Social Interaction Reward. International Journal of Molecular Sciences, 22(1), 345. https://doi.org/10.3390/ijms22010345