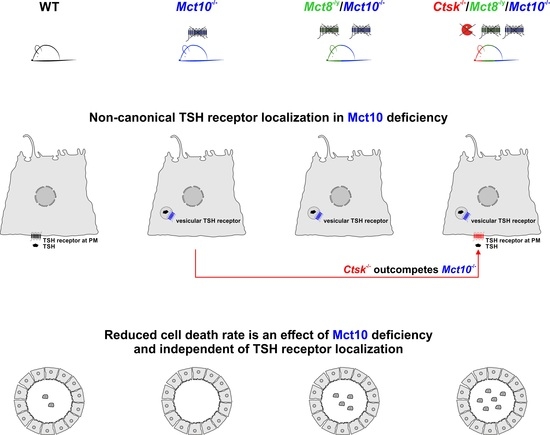
The Amino Acid Transporter Mct10/Tat1 Is Important to Maintain the TSH Receptor at Its Canonical Basolateral Localization and Assures Regular Turnover of Thyroid Follicle Cells in Male Mice
Abstract
:1. Introduction
2. Results
2.1. Serum TSH Concentrations in Mice Lacking Cathepsin K and TH Transporters
2.2. Localization of TSH Receptors in Thyroid Glands of Combined Cathepsin K and/or TH Transporter Deficiency
2.3. Assessing Endocytic Down-Regulation of TSH Receptors in Combined Cathepsin K and TH Transporter Deficiencies
2.4. Sub-Follicular Cathepsin Distribution Is Unaltered in Combined Cathepsin K- and TH Transporter-Deficient Mice
2.5. Measurement of Epithelial Heights in Cathepsin K and/or TH Transporter Deficiency
2.6. Assessing Thyroid Gland Architecture by Morphometry in Combined Cathepsin K and/or TH Transporter Deficiencies
2.7. Enumerating Thyrocytes in Cathepsin K and/or TH Transporter Deficiency Reveals Absence of Mct10 as a Thyrocyte Survival Factor
3. Discussion
3.1. Heteromerization of TSH Receptor and Mct8 Regulates Thyroid Gland Activity and Disruption in This Interaction Leads to Altered Thyroid Functional States
3.2. TSH Receptor Signaling Is Not Only Limited to the Cell Surface but also Persists within Intracellular Compartments
3.3. Altered TSH Receptor Signaling Does Not Necessarily Lead to Altered Thyroid Gland Morphology, but Absence of Mct10 Affects Thyrocyte Turnover
4. Materials and Methods
4.1. Murine Models
4.2. Serum Preparation, Dissection of Thyroid Tissue and Cryosectioning
4.3. Indirect Immunofluorescence
4.4. Image Acquisition
4.5. Automated Image Analysis
4.5.1. Fluorescence Intensity Measurements of Immunostained Thyroid Cryosections (Pipeline P1)
4.5.2. Morphological Analysis of the Thyroid Gland (Pipelines P2 and P3)
4.6. Preparation of Thyroid Tissue Lysate
4.7. SDS-PAGE and Immunoblotting
4.8. Measurement of Serum TSH Concentrations
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brix, K.; Qatato, M.; Szumska, J.; Venugopalan, V.; Rehders, M. Thyroglobulin Storage, Processing and Degradation for Thyroid Hormone Liberation. In The Thyroid and Its Diseases; Luster, M., Duntas, L.H., Wartofsky, L., Eds.; Springer International Publishing: New York, NY, USA, 2019; pp. 25–48. [Google Scholar] [CrossRef]
- Groeneweg, S.; van Geest, F.S.; Peeters, R.P.; Heuer, H.; Visser, W.E. Thyroid Hormone Transporters. Endocr. Rev. 2020, 41, 146–201. [Google Scholar] [CrossRef] [PubMed]
- Brix, K.; Szumska, J.; Weber, J.; Qatato, M.; Venugopalan, V.; Al-Hashimi, A.; Rehders, M. Auto-Regulation of the Thyroid Gland Beyond Classical Pathways. Exp. Clin. Endocrinol. Diabetes Off. J. Ger. Soc. Endocrinol. Ger. Diabetes Assoc. 2020, 128, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Fliers, E.; Kalsbeek, A.; Boelen, A. Beyond the fixed setpoint of the hypothalamus-pituitary-thyroid axis. Eur. J. Endocrinol. 2014, 171, R197–R208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortiga-Carvalho, T.M.; Chiamolera, M.I.; Pazos-Moura, C.C.; Wondisford, F.E. Hypothalamus-Pituitary-Thyroid Axis. Compr. Physiol. 2016, 6, 1387–1428. [Google Scholar] [CrossRef] [PubMed]
- Venugopalan, V.; Al-Hashimi, A.; Rehders, M.; Golchert, J.; Reinecke, V.; Homuth, G.; Völker, U.; Manirajah, M.; Touzani, A.; Weber, J.; et al. The Thyroid Hormone Transporter Mct8 Restricts Cathepsin-Mediated Thyroglobulin Processing in Male Mice through Thyroid Auto-Regulatory Mechanisms That Encompass Autophagy. Int. J. Mol. Sci. 2021, 22, 462. [Google Scholar] [CrossRef]
- Qatato, M.; Szumska, J.; Skripnik, V.; Rijntjes, E.; Köhrle, J.; Brix, K. Canonical TSH Regulation of Cathepsin-Mediated Thyroglobulin Processing in the Thyroid Gland of Male Mice Requires Taar1 Expression. Front. Pharmacol. 2018, 9, 221. [Google Scholar] [CrossRef] [Green Version]
- Weber, J.; McInnes, J.; Kizilirmak, C.; Rehders, M.; Qatato, M.; Wirth, E.K.; Schweizer, U.; Verrey, F.; Heuer, H.; Brix, K. Interdependence of thyroglobulin processing and thyroid hormone export in the mouse thyroid gland. Eur. J. Cell Biol. 2017, 96, 440–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Führer, D.; Brix, K.; Biebermann, H. Understanding the Healthy Thyroid State in 2015. Eur. Thyroid J. 2015, 4, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Wirth, E.K.; Sheu, S.Y.; Chiu-Ugalde, J.; Sapin, R.; Klein, M.O.; Mossbrugger, I.; Quintanilla-Martinez, L.; de Angelis, M.H.; Krude, H.; Riebel, T.; et al. Monocarboxylate transporter 8 deficiency: Altered thyroid morphology and persistent high triiodothyronine/thyroxine ratio after thyroidectomy. Eur. J. Endocrinol. 2011, 165, 555–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, J.; Mayerl, S.; Visser, T.J.; Darras, V.M.; Boelen, A.; Frappart, L.; Mariotta, L.; Verrey, F.; Heuer, H. Tissue-specific alterations in thyroid hormone homeostasis in combined Mct10 and Mct8 deficiency. Endocrinology 2014, 155, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Kero, J.; Ahmed, K.; Wettschureck, N.; Tunaru, S.; Wintermantel, T.; Greiner, E.; Schütz, G.; Offermanns, S. Thyrocyte-specific Gq/G11 deficiency impairs thyroid function and prevents goiter development. J. Clin. Investig. 2007, 117, 2399–2407. [Google Scholar] [CrossRef] [Green Version]
- Friedrichs, B.; Tepel, C.; Reinheckel, T.; Deussing, J.; von Figura, K.; Herzog, V.; Peters, C.; Saftig, P.; Brix, K. Thyroid functions of mouse cathepsins B, K, and L. J. Clin. Investig. 2003, 111, 1733–1745. [Google Scholar] [CrossRef] [Green Version]
- Dauth, S.; Rakov, H.; Sîrbulescu, R.F.; Ilieş, I.; Weber, J.; Batbajar Dugershaw, B.; Braun, D.; Rehders, M.; Wirth, E.K.; Führer, D.; et al. Function of Cathepsin K in the Central Nervous System of Male Mice is Independent of Its Role in the Thyroid Gland. Cell. Mol. Neurobiol. 2020, 40, 695–710. [Google Scholar] [CrossRef]
- Trajkovic, M.; Visser, T.J.; Mittag, J.; Horn, S.; Lukas, J.; Darras, V.M.; Raivich, G.; Bauer, K.; Heuer, H. Abnormal thyroid hormone metabolism in mice lacking the monocarboxylate transporter 8. J. Clin. Investig. 2007, 117, 627–635. [Google Scholar] [CrossRef] [Green Version]
- Müller, J.; Heuer, H. Understanding the hypothalamus-pituitary-thyroid axis in mct8 deficiency. Eur. Thyroid J. 2012, 1, 72–79. [Google Scholar] [CrossRef] [Green Version]
- Calebiro, D.; Nikolaev, V.O.; Gagliani, M.C.; de Filippis, T.; Dees, C.; Tacchetti, C.; Persani, L.; Lohse, M.J. Persistent cAMP-Signals Triggered by Internalized G-Protein–Coupled Receptors. PLoS Biol. 2009, 7, e1000172. [Google Scholar] [CrossRef] [Green Version]
- Calebiro, D.; Nikolaev, V.O.; Persani, L.; Lohse, M.J. Signaling by internalized G-protein-coupled receptors. Trends Pharm. Sci 2010, 31, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Godbole, A.; Lyga, S.; Lohse, M.J.; Calebiro, D. Internalized TSH receptors en route to the TGN induce local Gs-protein signaling and gene transcription. Nat. Commun. 2017, 8, 443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Latif, R.; Graves, P.; Davies, T.F. Oligomerization of the human thyrotropin receptor: Fluorescent protein-tagged hTSHR reveals post-translational complexes. J. Biol. Chem. 2001, 276, 45217–45224. [Google Scholar] [CrossRef] [Green Version]
- Davies, T.; Marians, R.; Latif, R. The TSH receptor reveals itself. J. Clin. Investig. 2002, 110, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.; Kleinau, G.; Rutz, C.; Zwanziger, D.; Khajavi, N.; Muller, A.; Rehders, M.; Brix, K.; Worth, C.L.; Fuhrer, D.; et al. Evidence of G-protein-coupled receptor and substrate transporter heteromerization at a single molecule level. Cell. Mol. Life Sci. 2018, 75, 2227–2239. [Google Scholar] [CrossRef] [PubMed]
- Brix, K.; Lemansky, P.; Herzog, V. Evidence for extracellularly acting cathepsins mediating thyroid hormone liberation in thyroid epithelial cells. Endocrinology 1996, 137, 1963–1974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linke, M.; Jordans, S.; Mach, L.; Herzog, V.; Brix, K. Thyroid stimulating hormone upregulates secretion of cathepsin B from thyroid epithelial cells. Biol. Chem. 2002, 383, 773–784. [Google Scholar] [CrossRef] [PubMed]
- Dumont, J.E.; Lamy, F.; Roger, P.; Maenhaut, C. Physiological and pathological regulation of thyroid cell proliferation and differentiation by thyrotropin and other factors. Physiol. Rev. 1992, 72, 667–697. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, M.; Fagman, H. Development of the thyroid gland. Development (Camb. Engl.) 2017, 144, 2123–2140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dumitrescu, A.M.; Liao, X.H.; Weiss, R.E.; Millen, K.; Refetoff, S. Tissue-specific thyroid hormone deprivation and excess in monocarboxylate transporter (mct) 8-deficient mice. Endocrinology 2006, 147, 4036–4043. [Google Scholar] [CrossRef] [Green Version]
- Allgeier, A.; Offermanns, S.; Van Sande, J.; Spicher, K.; Schultz, G.; Dumont, J.E. The human thyrotropin receptor activates G-proteins Gs and Gq/11. J. Biol. Chem. 1994, 269, 13733–13735. [Google Scholar] [CrossRef]
- Allen, M.D.; Neumann, S.; Gershengorn, M.C. Occupancy of both sites on the thyrotropin (TSH) receptor dimer is necessary for phosphoinositide signaling. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2011, 25, 3687–3694. [Google Scholar] [CrossRef] [Green Version]
- Botta, R.; Lisi, S.; Pinchera, A.; Giorgi, F.; Marcocci, C.; Taddei, A.R.; Fausto, A.M.; Bernardini, N.; Ippolito, C.; Mattii, L.; et al. Sortilin is a putative postendocytic receptor of thyroglobulin. Endocrinology 2009, 150, 509–518. [Google Scholar] [CrossRef] [Green Version]
- Giordano, C.; Richiusa, P.; Bagnasco, M.; Salmaso, C.; Pizzolanti, G.; Galluzzo, A. Thyrocytes—Not innocent bystanders in autoimmune disease. Nat. Immunol. 2001, 2, 183. [Google Scholar] [CrossRef]
- Lin, J.D. The role of apoptosis in autoimmune thyroid disorders and thyroid cancer. BMJ 2001, 322, 1525–1527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stassi, G.; De Maria, R. Autoimmune thyroid disease: New models of cell death in autoimmunity. Nat. Rev. Immunol. 2002, 2, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Palazzo, F.F.; Hammond, L.J.; Goode, A.W.; Mirakian, R. Death of the autoimmune thyrocyte: Is it pushed or does it jump? Thyroid Off. J. Am. Thyroid Assoc. 2000, 10, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Andrikoula, M.; Tsatsoulis, A. The role of Fas-mediated apoptosis in thyroid disease. Eur. J. Endocrinol. 2001, 144, 561–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsatsoulis, A. The role of apoptosis in thyroid disease. Minerva Med. 2002, 93, 169–180. [Google Scholar] [PubMed]
- McQuin, C.; Goodman, A.; Chernyshev, V.; Kamentsky, L.; Cimini, B.A.; Karhohs, K.W.; Doan, M.; Ding, L.; Rafelski, S.M.; Thirstrup, D.; et al. CellProfiler 3.0: Next-generation image processing for biology. PLoS Biol. 2018, 16, e2005970. [Google Scholar] [CrossRef] [Green Version]
- Neuhoff, V.; Philipp, K.; Zimmer, H.G.; Mesecke, S. A simple, versatile, sensitive and volume-independent method for quantitative protein determination which is independent of other external influences. Hoppe-Seyler’s Z. Physiol. Chem. 1979, 360, 1657–1670. [Google Scholar] [CrossRef] [PubMed]
- Abdulrahman, R.M.; Boon, M.R.; Sips, H.C.; Guigas, B.; Rensen, P.C.; Smit, J.W.; Hovens, G.C. Impact of Metformin and compound C on NIS expression and iodine uptake in vitro and in vivo: A role for CRE in AMPK modulation of thyroid function. Thyroid Off. J. Am. Thyroid Assoc. 2014, 24, 78–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, X.-Y.; Shi, X.-J.; Hu, A.; Wang, J.-Q.; Ding, Y.; Jiang, W.; Sun, M.; Zhao, X.; Luo, J.; Qi, W.; et al. Feeding induces cholesterol biosynthesis via the mTORC1–USP20–HMGCR axis. Nature 2020, 588, 479–484. [Google Scholar] [CrossRef]
- Kunath, A.; Weiner, J.; Krause, K.; Rehders, M.; Pejkovska, A.; Gericke, M.; Biniossek, M.L.; Dommel, S.; Kern, M.; Ribas-Latre, A.; et al. Role of Kallikrein 7 in Body Weight and Fat Mass Regulation. Biomedicines 2021, 9, 131. [Google Scholar] [CrossRef]








| Parameter | Proxy for Gαq or Gαs | Ctsk−/− | Mct10−/− | Mct8−/y | Mct8−/y/ Mct10−/− | Ctsk−/−/ Mct10−/− | Ctsk−/−/ Mct8−/y | Ctsk−/−/ Mct8−/y/ Mct10−/− |
|---|---|---|---|---|---|---|---|---|
| Serum TSH | = | = [11] | = ↑ [10,11,27] | ↑ [11] | = | = | = | |
| TSH receptor localization | basolateral | vesicular | basolateral | vesicular | basolateral | basolateral | basolateral and vesicular | |
| EExt | Gαq | = [14] | = | = | ↑ | = | = | ↑ |
| extra- vs. intracellular cathepsins | Gαq | ↑ [13] | = [8] | = [8] | = [8] | = | = | = |
| Tg degradation | Gαq | = [6] | = [8] | ↑ [8] | ↑ [8] | = [6] | ↑ [6] | ↑ [6] |
| Tg protein | Gαs | = [6] | ↓ [6] | = [6] | = [6] | |||
| Tg cross-linkage | Gαs | = [6] | = [8] | ↓ [8] | ↓ [8] | = [6] | = [6] | = [6] |
| Tg glycosylation | Gαs | = [6] | ↓ [6] | ↓ [6] | ↓ [6] | |||
| cell. Cath B | Gαs | = [13] | ↑ [8] | ↑ [8] | ↑ [8] | = | ↑ | ↑ |
| cell. Cath D | Gαs | = [13] | = [8] | = [8] | ↑ [8] | = | ↑ | ↑ |
| cell. Cath L | Gαs | ↑ [13] | ↑ [8] | ↑ [8] | ↑ [8] | = | ↑ | ↑ |
| Follicle no. | Gαs | = [14] | = | = | = | = | ↑ | = |
| Follicle area | Gαs | = [14] | = | ↑ | ↑ | = | = | ↑ |
| Lumen area | Gαs | = [14] | = | ↑ | ↑ | = | = | ↑ |
| TC area | Gαs | = | ↓ | ↑ | ↑ | = | = | ↑ |
| Cell no. per 1000 µm2 | Gαs | = | ↑ | ↓ | ↓ | = | = | ↓ |
| Cell Death | Gαs | = | ↓ | ↑ | ↑ | = | = | ↑ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venugopalan, V.; Al-Hashimi, A.; Weber, J.; Rehders, M.; Qatato, M.; Wirth, E.K.; Schweizer, U.; Heuer, H.; Verrey, F.; Brix, K. The Amino Acid Transporter Mct10/Tat1 Is Important to Maintain the TSH Receptor at Its Canonical Basolateral Localization and Assures Regular Turnover of Thyroid Follicle Cells in Male Mice. Int. J. Mol. Sci. 2021, 22, 5776. https://doi.org/10.3390/ijms22115776
Venugopalan V, Al-Hashimi A, Weber J, Rehders M, Qatato M, Wirth EK, Schweizer U, Heuer H, Verrey F, Brix K. The Amino Acid Transporter Mct10/Tat1 Is Important to Maintain the TSH Receptor at Its Canonical Basolateral Localization and Assures Regular Turnover of Thyroid Follicle Cells in Male Mice. International Journal of Molecular Sciences. 2021; 22(11):5776. https://doi.org/10.3390/ijms22115776
Chicago/Turabian StyleVenugopalan, Vaishnavi, Alaa Al-Hashimi, Jonas Weber, Maren Rehders, Maria Qatato, Eva K. Wirth, Ulrich Schweizer, Heike Heuer, François Verrey, and Klaudia Brix. 2021. "The Amino Acid Transporter Mct10/Tat1 Is Important to Maintain the TSH Receptor at Its Canonical Basolateral Localization and Assures Regular Turnover of Thyroid Follicle Cells in Male Mice" International Journal of Molecular Sciences 22, no. 11: 5776. https://doi.org/10.3390/ijms22115776
APA StyleVenugopalan, V., Al-Hashimi, A., Weber, J., Rehders, M., Qatato, M., Wirth, E. K., Schweizer, U., Heuer, H., Verrey, F., & Brix, K. (2021). The Amino Acid Transporter Mct10/Tat1 Is Important to Maintain the TSH Receptor at Its Canonical Basolateral Localization and Assures Regular Turnover of Thyroid Follicle Cells in Male Mice. International Journal of Molecular Sciences, 22(11), 5776. https://doi.org/10.3390/ijms22115776